
A blood type is a classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells (RBCs). These antigens may be proteins, carbohydrates, glycoproteins, or glycolipids, depending on the blood group system. Some of these antigens are also present on the surface of other types of cells of various tissues. Several of these red blood cell surface antigens can stem from one allele and collectively form a blood group system.

The human leukocyte antigen (HLA) system or complex is a complex of genes on chromosome 6 in humans which encode cell-surface proteins responsible for regulation of the immune system. The HLA system is also known as the human version of the major histocompatibility complex (MHC) found in many animals.
Glycophorin C plays a functionally important role in maintaining erythrocyte shape and regulating membrane material properties, possibly through its interaction with protein 4.1. Moreover, it has previously been shown that membranes deficient in protein 4.1 exhibit decreased content of glycophorin C. It is also an integral membrane protein of the erythrocyte and acts as the receptor for the Plasmodium falciparum protein PfEBP-2.

Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234, is a protein that in humans is encoded by the ACKR1 gene.

The ABO blood group system is used to denote the presence of one, both, or neither of the A and B antigens on erythrocytes. For human blood transfusions, it is the most important of the 44 different blood type classification systems currently recognized by the International Society of Blood Transfusions (ISBT) as of December 2022. A mismatch in this, or any other serotype, can cause a potentially fatal adverse reaction after a transfusion, or an unwanted immune response to an organ transplant. The associated anti-A and anti-B antibodies are usually IgM antibodies, produced in the first years of life by sensitization to environmental substances such as food, bacteria, and viruses.
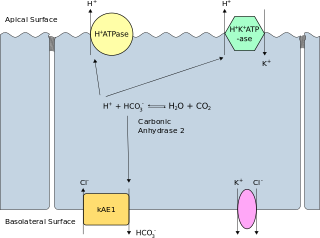
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
Genetics and archaeogenetics of South Asia is the study of the genetics and archaeogenetics of the ethnic groups of South Asia. It aims at uncovering these groups' genetic histories. The geographic position of the Indian subcontinent makes its biodiversity important for the study of the early dispersal of anatomically modern humans across Asia.
The Kidd antigen system are proteins found in the Kidd's blood group, which act as antigens, i.e., they have the ability to produce antibodies under certain circumstances. The Jk antigen is found on a protein responsible for urea transport in the red blood cells and the kidney. They are important in transfusion medicine. People with two Jk(a) antigens, for instance, may form antibodies against donated blood containing two Jk(b) antigens. This can lead to hemolytic anemia, in which the body destroys the transfused blood, leading to low red blood cell counts. Another disease associated with the Jk antigen is hemolytic disease of the newborn, in which a pregnant woman's body creates antibodies against the blood of her fetus, leading to destruction of the fetal blood cells. Hemolytic disease of the newborn associated with Jk antibodies is typically mild, though fatal cases have been reported.

The Rh blood group system is a human blood group system. It contains proteins on the surface of red blood cells. After the ABO blood group system, it is the most likely to be involved in transfusion reactions. The Rh blood group system consisted of 49 defined blood group antigens in 2005. As of 2023, there are over 50 antigens among which the five antigens D, C, c, E, and e are the most important. There is no d antigen. Rh(D) status of an individual is normally described with a positive (+) or negative (−) suffix after the ABO type. The terms Rh factor, Rh positive, and Rh negative refer to the Rh(D) antigen only. Antibodies to Rh antigens can be involved in hemolytic transfusion reactions and antibodies to the Rh(D) and Rh antigens confer significant risk of hemolytic disease of the fetus and newborn
HLA DR3-DQ2 is double serotype that specifically recognizes cells from individuals who carry a multigene HLA DR, DQ haplotype. Certain HLA DR and DQ genes have known involvement in autoimmune diseases. DR3-DQ2, a multigene haplotype, stands out in prominence because it is a factor in several prominent diseases, namely coeliac disease and juvenile diabetes. In coeliac disease, the DR3-DQ2 haplotype is associated with highest risk for disease in first degree relatives, highest risk is conferred by DQA1*0501:DQB1*0201 homozygotes and semihomozygotes of DQ2, and represents the overwhelming majority of risk. HLA DR3-DQ2 encodes DQ2.5cis isoform of HLA-DQ, this isoform is described frequently as 'the DQ2 isoform', but in actuality there are two major DQ2 isoform. The DQ2.5 isoform, however, is many times more frequently associated with autoimmune disease, and as a result to contribution of DQ2.2 is often ignored.

HLA-DQ8 (DQ8) is a human leukocyte antigen serotype within the HLA-DQ (DQ) serotype group. DQ8 is a split antigen of the DQ3 broad antigen. DQ8 is determined by the antibody recognition of β8 and this generally detects the gene product of DQB1*0302.
HLA-B46 (B46) is an HLA-B serotype. The serotype identifies the gene products of HLA-B*4601 allele. B*4601 resulted from a rare, interlocus, gene conversion between B62, probably B*1501, and a HLA-C allele. B*4601 is the most common HLA-B allele that does not have an origin within Africa, and estimated 400 million people in Eastern Asia carry a B46 allele. When found B*4601 segregates with only 2 HLA-Cw alleles, A limited number of HLA-A and HLA-DRB1 alleles suggesting that the allele recently expanded from a limited sized group within SE Asia. Extremely low frequencies outside of Eastern Asia are indicators of a recent expansion of B46 from a recently small population. The frequency distribution suggests the ancestral B46 population was in SE China, or, potentially Burma. B46 in Asia correlates with wet-rice farming. The exceptions are notable, it has been found in the Nivkhi on north-eastern Sakalin Island, the Ainu, and the Nivkhi-related (genetically) Tlinglet population of Alaska at trace levels.

Glycophorin A (MNS blood group), also known as GYPA, is a protein which in humans is encoded by the GYPA gene. GYPA has also recently been designated CD235a (cluster of differentiation 235a).
HLA A30-Cw5-B18-DR3-DQ2 (A30::DQ2) is a multigene haplotype that extends across a majority of the major histocompatibility complex on human chromosome 6. A multigene haplotype is a set of inherited alleles covering several genes, or gene-alleles. Long haplotypes, like A30::DQ2, are generally the result of descent by common ancestry. As haplotypes increase in size, Chromosomal recombination fragments them in a generation dependent process.
The genetic history of the Indigenous peoples of the Americas is divided into two distinct periods: the initial peopling of the Americas during about 20,000 to 14,000 years ago, and European contact, after about 500 years ago. The first period of Indigenous American genetic history is the determinant factor for the number of genetic lineages, zygosity mutations and founding haplotypes present in today's Indigenous American populations.
This list concerns blood type distribution between countries and regions. Blood type is a classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells (RBCs). These antigens may be proteins, carbohydrates, glycoproteins, or glycolipids, depending on the blood group system.
Secretor status refers to the presence or absence of water-soluble ABO blood group antigens in a person's bodily fluids, such as saliva, tears, breast milk, urine, and semen. People who secrete these antigens in their bodily fluids are referred to as secretors, while people who do not are termed non-secretors. Secretor status is controlled by the FUT2 gene, and the secretor phenotype is inherited in an autosomal dominant manner, being expressed by individuals who have at least one functioning copy of the gene. The non-secretor phenotype (se) is a recessive trait. Approximately 80% of Caucasian people are secretors, while 20% are non-secretors. Non-secretors have reduced susceptibility to the most common strains of norovirus. Expression of the antigens in the Lewis blood group is also affected by secretor status: non-secretors cannot produce the Le(b) antigen.
The Vel blood group is a human blood group that has been implicated in hemolytic transfusion reactions. The blood group consists of a single antigen, the high-frequency Vel antigen, which is expressed on the surface of red blood cells. Individuals are typed as Vel-positive or Vel-negative depending on the presence of this antigen. The expression of the antigen in Vel-positive individuals is highly variable and can range from strong to weak. Individuals with the rare Vel-negative blood type develop anti-Vel antibodies when exposed to Vel-positive blood, which can cause transfusion reactions on subsequent exposures.
The Junior blood group system is a human blood group defined by the presence or absence of the Jr(a) antigen, a high-frequency antigen that is found on the red blood cells of most individuals. People with the rare Jr(a) negative blood type can develop anti-Jr(a) antibodies, which may cause transfusion reactions and hemolytic disease of the newborn on subsequent exposures. Jr(a) negative blood is most common in people of Japanese heritage.
The Sid blood group system is a human blood group defined by the presence or absence of the Sd(a) antigen on a person's red blood cells. About 96% of people are positive for the Sd(a) antigen, which is inherited as a dominant trait. Among Sd(a) positive individuals, the expression of the antigen ranges from extremely weak to extremely strong. Very strong expression of the antigen is referred to as a Sd(a++) phenotype. In addition to being expressed on red blood cells, Sd(a) is secreted in bodily fluids such as saliva and breast milk, and is found in the highest concentrations in urine. Urine testing is considered the most reliable method for determining a person's Sid blood type.







