Related Research Articles

A blood type is a classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells (RBCs). These antigens may be proteins, carbohydrates, glycoproteins, or glycolipids, depending on the blood group system. Some of these antigens are also present on the surface of other types of cells of various tissues. Several of these red blood cell surface antigens can stem from one allele and collectively form a blood group system.
Humoral immunity is the aspect of immunity that is mediated by macromolecules – including secreted antibodies, complement proteins, and certain antimicrobial peptides – located in extracellular fluids. Humoral immunity is named so because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Humoral immunity is also referred to as antibody-mediated immunity.

Hemolytic anemia or haemolytic anaemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells (RBCs), either in the blood vessels or elsewhere in the human body (extravascular). This most commonly occurs within the spleen, but also can occur in the reticuloendothelial system or mechanically. Hemolytic anemia accounts for 5% of all existing anemias. It has numerous possible consequences, ranging from general symptoms to life-threatening systemic effects. The general classification of hemolytic anemia is either intrinsic or extrinsic. Treatment depends on the type and cause of the hemolytic anemia.

Hemolytic disease of the newborn, also known as hemolytic disease of the fetus and newborn, HDN, HDFN, or erythroblastosis fetalis, is an alloimmune condition that develops in a fetus at or around birth, when the IgG molecules produced by the mother pass through the placenta. Among these antibodies are some which attack antigens on the red blood cells in the fetal circulation, breaking down and destroying the cells. The fetus can develop reticulocytosis and anemia. The intensity of this fetal disease ranges from mild to very severe, and fetal death from heart failure can occur. When the disease is moderate or severe, many erythroblasts are present in the fetal blood, earning these forms of the disease the name erythroblastosis fetalis.
The direct and indirect Coombs tests, also known as antiglobulin test (AGT), are blood tests used in immunohematology. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells they can cause anemia; this test can help clarify the condition. The indirect Coombs test detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells; the test can be carried out to diagnose reactions to a blood transfusion.
Alloimmunity is an immune response to nonself antigens from members of the same species, which are called alloantigens or isoantigens. Two major types of alloantigens are blood group antigens and histocompatibility antigens. In alloimmunity, the body creates antibodies against the alloantigens, attacking transfused blood, allotransplanted tissue, and even the fetus in some cases. Alloimmune (isoimmune) response results in graft rejection, which is manifested as deterioration or complete loss of graft function. In contrast, autoimmunity is an immune response to the self's own antigens. Alloimmunization (isoimmunization) is the process of becoming alloimmune, that is, developing the relevant antibodies for the first time.
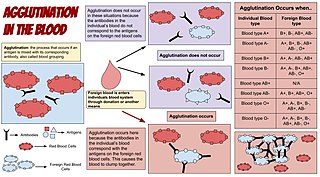
Agglutination is the clumping of particles. The word agglutination comes from the Latin agglutinare.
Autoimmune hemolytic anemia (AIHA) is an autoimmune disorder which occurs when antibodies directed against the person's own red blood cells (RBCs) cause them to burst (lyse), leading to an insufficient number of oxygen-carrying red blood cells in circulation (anemia). The lifetime of the RBCs is reduced from the normal 100–120 days to just a few days in serious cases. The intracellular components of the RBCs are released into the circulating blood and into tissues, leading to some of the characteristic symptoms of this condition. The antibodies are usually directed against high-incidence antigens, therefore they also commonly act on allogenic RBCs. AIHA is a relatively rare condition, with an incidence of 5–10 cases per 1 million persons per year in the warm-antibody type and 0.45 to 1.9 cases per 1 million persons per year in the cold-antibody type. Autoimmune hemolysis might be a precursor of later onset systemic lupus erythematosus.
Paroxysmal cold hemoglobinuria (PCH) or Donath–Landsteiner hemolytic anemia (DLHA) is an autoimmune hemolytic anemia featured by complement-mediated intravascular hemolysis after cold exposure. It can present as an acute non-recurrent postinfectious event in children, or chronic relapsing episodes in adults with hematological malignancies or tertiary syphilis. Described by Julius Donath (1870–1950) and Karl Landsteiner (1868–1943) in 1904, PCH is one of the first clinical entities recognized as an autoimmune disorder.
In ABO hemolytic disease of the newborn maternal IgG antibodies with specificity for the ABO blood group system pass through the placenta to the fetal circulation where they can cause hemolysis of fetal red blood cells which can lead to fetal anemia and HDN. In contrast to Rh disease, about half of the cases of ABO HDN occur in a firstborn baby and ABO HDN does not become more severe after further pregnancies.
Hemolytic disease of the newborn (anti-Kell1) is the second most common cause of severe hemolytic disease of the newborn (HDN) after Rh disease. Anti-Kell1 is becoming relatively more important as prevention of Rh disease is also becoming more effective.
The Kidd antigen system are proteins found in the Kidd's blood group, which act as antigens, i.e., they have the ability to produce antibodies under certain circumstances. The Jk antigen is found on a protein responsible for urea transport in the red blood cells and the kidney. They are important in transfusion medicine. People with two Jk(a) antigens, for instance, may form antibodies against donated blood containing two Jk(b) antigens. This can lead to hemolytic anemia, in which the body destroys the transfused blood, leading to low red blood cell counts. Another disease associated with the Jk antigen is hemolytic disease of the newborn, in which a pregnant woman's body creates antibodies against the blood of her fetus, leading to destruction of the fetal blood cells. Hemolytic disease of the newborn associated with Jk antibodies is typically mild, though fatal cases have been reported.
Type II hypersensitivity, in the Gell and Coombs classification of allergic reactions, is an antibody mediated process in which IgG and IgM antibodies are directed against antigens on cells or extracellular material. This subsequently leads to cell lysis, tissue damage or loss of function through mechanisms such as
- complement activation via the classical complement pathway
- Antibody-dependent cellular cytotoxicity or
- anti-receptor activity.
Hematologic diseases are disorders which primarily affect the blood and blood-forming organs. Hematologic diseases include rare genetic disorders, anemia, HIV, sickle cell disease and complications from chemotherapy or transfusions.
This page is currently under construction.
Rh factor testing, also known as Rhesus factor testing, is the procedure of determining the Rhesus D status of an individual.
The Vel blood group is a human blood group that has been implicated in hemolytic transfusion reactions. The blood group consists of a single antigen, the high-frequency Vel antigen, which is expressed on the surface of red blood cells. Individuals are typed as Vel-positive or Vel-negative depending on the presence of this antigen. The expression of the antigen in Vel-positive individuals is highly variable and can range from strong to weak. Individuals with the rare Vel-negative blood type develop anti-Vel antibodies when exposed to Vel-positive blood, which can cause transfusion reactions on subsequent exposures.
The Junior blood group system is a human blood group defined by the presence or absence of the Jr(a) antigen, a high-frequency antigen that is found on the red blood cells of most individuals. People with the rare Jr(a) negative blood type can develop anti-Jr(a) antibodies, which may cause transfusion reactions and hemolytic disease of the newborn on subsequent exposures. Jr(a) negative blood is most common in people of Japanese heritage.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.

The monocyte monolayer assay (MMA) is used to determine the clinical significance of alloantibodies produced by blood transfusion recipients. The assay is used to assess the potential for intravascular hemolysis when incompatible cellular blood products are transfused to the anemic patient. When donor cells possess substances that are not produced by the recipient, the recipient's immune system produces antibodies against the substance; these are called alloantibodies. Specific white blood cells, called monocytes, are tasked with ingesting foreign material and become activated during certain inflammatory events. These activated monocytes come in contact with antibody-sensitized red blood cells (RBC) and may or may not exhibit phagocytosis (ingestion) and destroy the donor red blood cells. If monocytes destroy the RBC, the antibody attached to those RBC is considered clinically significant.
References
- ↑ Covin RB, Evans KS, Olshock R, Thompson HW (2020). "Acute hemolytic transfusion reaction caused by anti-Coa". Immunohematology. 17 (2): 45–9. doi: 10.21307/immunohematology-2019-543 . PMID 15373591. S2CID 235243237.
- ↑ Hoffbrand, A. V.; P.A.H. Moss; J.E. Pettit (2006). Essential Haematology: 5th Edition. Blackwell Publishing. ISBN 1-4051-3649-9.
- ↑ Molthan L, Matulewicz TJ, Bansal-Carver B, Benz EJ (1984). "An immediate hemolytic transfusion reaction due to anti-C and a delayed hemolytic transfusion reaction due to anti-Ce+e: hemoglobinemia, hemoglobinuria and transient impaired renal function". Vox Sang. 47 (5): 348–53. doi:10.1111/j.1423-0410.1984.tb04138.x. PMID 6438912. S2CID 41434530.
- 1 2 3 4 5 6 7 8 9 Caligiuri, Michael; Levi, Marcel M.; Kaushansky, Kenneth; Lichtman, Marshall A.; Prchal, Josef; Burns, Linda J.; Press, Oliver W. (2015-12-23). Williams Hematology, 9E. McGraw-Hill Education. ISBN 9780071833004.
- 1 2 3 Jameson, J. Larry; Kasper, Dennis L.; Longo, Dan L.; Fauci, Anthony S.; Hauser, Stephen L.; Loscalzo, Joseph (2018-08-13). Harrison's principles of internal medicine (20th ed.). New York. ISBN 9781259644030. OCLC 1029074059.
{{cite book}}: CS1 maint: location missing publisher (link) - 1 2 3 4 5 6 7 8 9 10 11 Panch, Sandhya R.; Montemayor-Garcia, Celina; Klein, Harvey G. (11 July 2019). "Hemolytic Transfusion Reactions". New England Journal of Medicine. 381 (2): 150–162. doi:10.1056/NEJMra1802338. PMID 31291517. S2CID 261097623.