
A blood type is a classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells (RBCs). These antigens may be proteins, carbohydrates, glycoproteins, or glycolipids, depending on the blood group system. Some of these antigens are also present on the surface of other types of cells of various tissues. Several of these red blood cell surface antigens can stem from one allele and collectively form a blood group system.

Duffy antigen/chemokine receptor (DARC), also known as Fy glycoprotein (FY) or CD234, is a protein that in humans is encoded by the ACKR1 gene.

Hemolytic disease of the newborn, also known as hemolytic disease of the fetus and newborn, HDN, HDFN, or erythroblastosis foetalis, is an alloimmune condition that develops in a fetus at or around birth, when the IgG molecules produced by the mother pass through the placenta. Among these antibodies are some which attack antigens on the red blood cells in the fetal circulation, breaking down and destroying the cells. The fetus can develop reticulocytosis and anemia. The intensity of this fetal disease ranges from mild to very severe, and fetal death from heart failure can occur. When the disease is moderate or severe, many erythroblasts are present in the fetal blood, earning these forms of the disease the name erythroblastosis fetalis.

The ABO blood group system is used to denote the presence of one, both, or neither of the A and B antigens on erythrocytes. For human blood transfusions, it is the most important of the 44 different blood type classification systems currently recognized by the International Society of Blood Transfusions (ISBT) as of December 2022. A mismatch in this, or any other serotype, can cause a potentially fatal adverse reaction after a transfusion, or an unwanted immune response to an organ transplant. The associated anti-A and anti-B antibodies are usually IgM antibodies, produced in the first years of life by sensitization to environmental substances such as food, bacteria, and viruses.
In ABO hemolytic disease of the newborn maternal IgG antibodies with specificity for the ABO blood group system pass through the placenta to the fetal circulation where they can cause hemolysis of fetal red blood cells which can lead to fetal anemia and HDN. In contrast to Rh disease, about half of the cases of ABO HDN occur in a firstborn baby and ABO HDN does not become more severe after further pregnancies.
Hemolytic disease of the newborn (anti-Kell1) is the second most common cause of severe hemolytic disease of the newborn (HDN) after Rh disease. Anti-Kell1 is becoming relatively more important as prevention of Rh disease is also becoming more effective.
Hemolytic disease of the newborn (anti-Rhc) can range from a mild to a severe disease. It is the third most common cause of severe HDN. Rh disease is the most common and hemolytic disease of the newborn (anti-Kell) is the second most common cause of severe HDN. It occurs more commonly in women who are Rh D negative.
The Kell antigen system is a human blood group system, that is, a group of antigens on the human red blood cell surface which are important determinants of blood type and are targets for autoimmune or alloimmune diseases which destroy red blood cells. The Kell antigens are K, k, Kpa, Kpb, Jsa and Jsb. The Kell antigens are peptides found within the Kell protein, a 93-kilodalton transmembrane zinc-dependent endopeptidase which is responsible for cleaving endothelin-3.
The Kidd antigen system are proteins found in the Kidd's blood group, which act as antigens, i.e., they have the ability to produce antibodies under certain circumstances. The Jk antigen is found on a protein responsible for urea transport in the red blood cells and the kidney. They are important in transfusion medicine. People with two Jk(a) antigens, for instance, may form antibodies against donated blood containing two Jk(b) antigens. This can lead to hemolytic anemia, in which the body destroys the transfused blood, leading to low red blood cell counts. Another disease associated with the Jk antigen is hemolytic disease of the newborn, in which a pregnant woman's body creates antibodies against the blood of her fetus, leading to destruction of the fetal blood cells. Hemolytic disease of the newborn associated with Jk antibodies is typically mild, though fatal cases have been reported.

The Rh blood group system is a human blood group system. It contains proteins on the surface of red blood cells. After the ABO blood group system, it is the most likely to be involved in transfusion reactions. The Rh blood group system consisted of 49 defined blood group antigens in 2005. As of 2023, there are over 50 antigens among which the five antigens D, C, c, E, and e are the most important. There is no d antigen. Rh(D) status of an individual is normally described with a positive (+) or negative (−) suffix after the ABO type. The terms Rh factor, Rh positive, and Rh negative refer to the Rh(D) antigen only. Antibodies to Rh antigens can be involved in hemolytic transfusion reactions and antibodies to the Rh(D) and Rh antigens confer significant risk of hemolytic disease of the fetus and newborn
hh, or the Bombay blood group, is a rare blood type. This blood phenotype was first discovered in Bombay by Dr. Y. M. Bhende in 1952. It is mostly found in the Indian sub-continent and Iran.
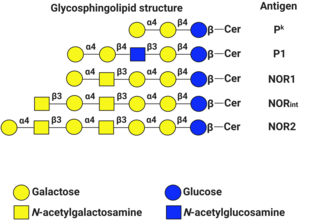
P1PK is a human blood group system based upon the A4GALT gene on chromosome 22. The P antigen was first described by Karl Landsteiner and Philip Levine in 1927. The P1PK blood group system consists of three glycosphingolipid antigens: Pk, P1 and NOR. In addition to glycosphingolipids, terminal Galα1→4Galβ structures are present on complex-type N-glycans. The GLOB antigen is now the member of the separate GLOB blood group system.
The Lewis antigen system is a human blood group system. It is based upon two genes on chromosome 19: FUT3, or Lewis gene; and FUT2, or Secretor gene. Both genes are expressed in glandular epithelia. FUT2 has a dominant allele which codes for an enzyme and a recessive allele which does not produce a functional enzyme. Similarly, FUT3 has a functional dominant allele (Le) and a non-functional recessive allele (le).

The Ii antigen system is a human blood group system based upon a gene on chromosome 6 and consisting of the I antigen and the i antigen. The I antigen is normally present on the cell membrane of red blood cells in all adults, while the i antigen is present in fetuses and newborns.
Hemolytic disease of the newborn (anti-RhE) is caused by the anti-RhE antibody of the Rh blood group system. The anti-RhE antibody can be naturally occurring, or arise following immune sensitization after a blood transfusion or pregnancy.

Rh blood group, D antigen also known as Rh polypeptide 1 (RhPI) or cluster of differentiation 240D (CD240D) is a protein that in humans is encoded by the RHD gene.
The Vel blood group is a human blood group that has been implicated in hemolytic transfusion reactions. The blood group consists of a single antigen, the high-frequency Vel antigen, which is expressed on the surface of red blood cells. Individuals are typed as Vel-positive or Vel-negative depending on the presence of this antigen. The expression of the antigen in Vel-positive individuals is highly variable and can range from strong to weak. Individuals with the rare Vel-negative blood type develop anti-Vel antibodies when exposed to Vel-positive blood, which can cause transfusion reactions on subsequent exposures.
The Lan blood group system is a human blood group defined by the presence or absence of the Lan antigen on a person's red blood cells. More than 99.9% of people are positive for the Lan antigen. Individuals with the rare Lan-negative blood type, which is a recessive trait, can produce an anti-Lan antibody when exposed to Lan-positive blood. Anti-Lan antibodies may cause transfusion reactions on subsequent exposures to Lan-positive blood, and have also been implicated in mild cases of hemolytic disease of the newborn. However, the clinical significance of the antibody is variable. The antigen was first described in 1961, and Lan was officially designated a blood group in 2012.
The Sid blood group system is a human blood group defined by the presence or absence of the Sd(a) antigen on a person's red blood cells. About 96% of people are positive for the Sd(a) antigen, which is inherited as a dominant trait. Among Sd(a) positive individuals, the expression of the antigen ranges from extremely weak to extremely strong. Very strong expression of the antigen is referred to as a Sd(a++) phenotype. In addition to being expressed on red blood cells, Sd(a) is secreted in bodily fluids such as saliva and breast milk, and is found in the highest concentrations in urine. Urine testing is considered the most reliable method for determining a person's Sid blood type.
The Augustine blood group system is a human blood group system. It includes four red blood cell surface glycoprotein antigens which are encoded by alleles of the gene SLC29A1.






