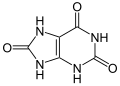
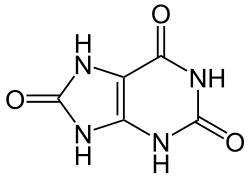
Contents
- Chemistry
- Biochemistry
- Water solubility
- Genetic and physiological diversity
- Primates
- Dogs
- Birds, reptiles and desert-dwelling mammals
- Invertebrates
- Bacteria
- Genetics
- Clinical significance and research
- High uric acid
- Low uric acid
- See also
- References
- External links
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 7,9-Dihydro-1H-purine-2,6,8(3H)-trione | |||
| Other names 2,6,8-Trioxypurine; 2,6,8-Trihydroxypurine; 2,6,8-Trioxopurine; 1H-Purine-2,6,8-trione | |||
| Identifiers | |||
3D model (JSmol) |
| ||
| 156158 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.655 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Uric+Acid | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| Properties | |||
| C5H4N4O3 | |||
| Molar mass | 168.112 g·mol−1 | ||
| Appearance | White crystals | ||
| Melting point | 300 °C (572 °F; 573 K) | ||
| 6 mg/100 mL (at 20 °C) | |||
| log P | −1.107 | ||
| Acidity (pKa) | 5.6 | ||
| Basicity (pKb) | 8.4 | ||
| −6.62×10−5 cm3 mol−1 | |||
| Thermochemistry | |||
Heat capacity (C) | 166.15 J K−1 mol−1 (at 24.0 °C) | ||
Std molar entropy (S⦵298) | 173.2 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) | −619.69 to −617.93 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) | −1921.2 to −1919.56 kJ mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. [1] High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.