Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals and chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid may act to inhibit these reactions. To balance oxidative stress, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione.

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.
The Bradford protein assay was developed by Marion M. Bradford in 1976. It is a quick and accurate spectroscopic analytical procedure used to measure the concentration of protein in a solution. The reaction is dependent on the amino acid composition of the measured proteins.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
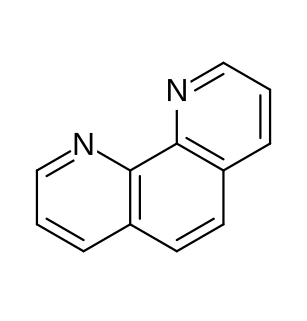
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, forming strong complexes with most metal ions. It is often sold as the monohydrate.
The FOX reagent, or ferrous oxidation−xylenol orange, is used to measure levels of hydrogen peroxide in biological systems. The reagent is incubated with the sample and absorbance of the product form after a series of oxidation reactions is then measured at a wavelength of 560 nm. The reagent itself is an aqueous solution of ferrous ammonium sulfate, sorbitol, sulfuric acid and xylenol orange.

In biochemistry, ABTS is a chemical compound used to observe the reaction kinetics of specific enzymes. A common use for it is in the enzyme-linked immunosorbent assay (ELISA) to detect the binding of molecules to each other.
Oxygen radical absorbance capacity (ORAC) was a method of measuring antioxidant capacities in biological samples in vitro. Because no physiological proof in vivo existed in support of the free-radical theory or that ORAC provided information relevant to biological antioxidant potential, it was withdrawn in 2012.
Barium ferrite, abbreviated BaFe, BaM, is the chemical compound with the formula BaFe12O19. This and related ferrite materials are components in magnetic stripe cards and loudspeaker magnets. BaFe is described as Ba2+(Fe3+)12(O2−)19. The Fe3+ centers are ferromagnetically coupled. This area of technology is usually considered to be an application of the related fields of materials science and solid state chemistry.
The Lowry protein assay is a biochemical assay for determining the total level of protein in a solution. The total protein concentration is exhibited by a color change of the sample solution in proportion to protein concentration, which can then be measured using colorimetric techniques. It is named for the biochemist Oliver H. Lowry who developed the reagent in the 1940s. His 1951 paper describing the technique is the most-highly cited paper ever in the scientific literature, cited over 300,000 times.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, also called the gallic acid equivalence method (GAE), is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants. It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.
The ferric chloride test is used to determine the presence of phenols in a given sample or compound. Enols, hydroxamic acids, oximes, and sulfinic acids give positive results as well. The bromine test is useful to confirm the result, although modern spectroscopic techniques are far superior in determining the identity of the unknown. The quantity of total phenols may be spectroscopically determined by the Folin–Ciocalteau assay.
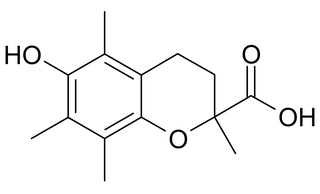
Trolox is a water-soluble analog of vitamin E sold by Hoffman-LaRoche. It is an antioxidant like vitamin E and it is used in biological or biochemical applications to reduce oxidative stress or damage.
The Trolox equivalent antioxidant capacity (TEAC) assay measures the antioxidant capacity of a given substance, as compared to the standard, Trolox. Most commonly, antioxidant capacity is measured using the ABTS Decolorization Assay. Other antioxidant capacity assays which use Trolox as a standard include the diphenylpicrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC) and ferric reducing ability of plasma (FRAP) assays. The TEAC assay is often used to measure the antioxidant capacity of foods, beverages and nutritional supplements.
The Trinder spot test is a diagnostic test used in medicine to determine exposure to salicylates, particularly to salicylic acid. The test employs the Trinder reagent which is mixed with a patient's urine. The colour change, resulting from the Trinder reaction, is immediate, enabling rapid bedside assessment.
A hydrolyzable tannin or pyrogallol-type tannin is a type of tannin that, on heating with hydrochloric or sulfuric acids, yields gallic or ellagic acids.

INT is a commonly used tetrazolium salt, similar to tetrazolium chloride that on reduction produces a red formazan dye that can be used for quantitative redox assays. It is also toxic to prokaryotes.
References
- ↑ Benzie, Iris F.F.; Strain, J.J. (1996). "The Ferric Reducing Ability of Plasma (FRAP) as a Measure of "Antioxidant Power": The FRAP Assay". Analytical Biochemistry. 239 (1): 70–6. doi:10.1006/abio.1996.0292. PMID 8660627.