Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals and chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid may act to inhibit these reactions. To balance oxidative stress, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione.

Five-spice powder is a spice mixture of five or more spices used predominantly in almost all branches of Chinese cuisine. It is also used in Hawaiian cuisine and Vietnamese cuisine. The five flavors of the spices refers to the five traditional Chinese elements.

The ton is a unit of measure. It has a long history and has acquired a number of meanings and uses over the years. It is used principally as a unit of weight. Its original use as a measurement of volume has continued in the capacity of cargo ships and in terms such as the freight ton. Recent specialized uses include the ton as a measure of energy and for truck classification. It is also a colloquial term, "ton" is the heaviest unit of weight typically used in colloquial speech. It is also used informally to mean a large amount of something, material or not.
Rancidification is the process of complete or incomplete oxidation or hydrolysis of fats and oils when exposed to air, light, or moisture or by bacterial action, resulting in unpleasant taste and odor. Specifically, it is the hydrolysis or autoxidation of fats into short-chain aldehydes, ketones and free fatty acids, which are objectionable in taste and odor. When these processes occur in food, undesirable odors and flavors can result.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Cyclic voltammetry (CV) is a type of potentiodynamic electrochemical measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

In biochemistry, ABTS is a chemical compound used to observe the reaction kinetics of specific enzymes. A common use for it is in the enzyme-linked immunosorbent assay (ELISA) to detect the binding of molecules to each other.
Oxygen radical absorbance capacity (ORAC) was a method of measuring antioxidant capacities in biological samples in vitro. Because no physiological proof in vivo existed in support of the free-radical theory or that ORAC provided information relevant to biological antioxidant potential, it was withdrawn in 2012.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, also called the gallic acid equivalence method (GAE), is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants. It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis. The Folin-Denis reagent is prepared by mixing sodium tungstate and phosphomolybdic acid in phosphoric acid. Folin-Ciocalteu reagent is just a modification of the Folin-Denis reagent. The modification consisted of the addition of lithium sulfate and bromine to the phosphotungstic-phosphomolybdic reagent.
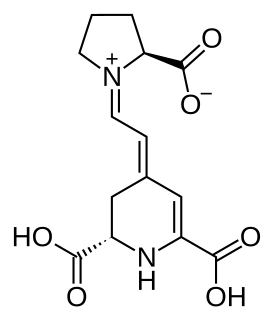
Indicaxanthin is a type of betaxanthin, a plant pigment present in beets, in Mirabilis jalapa flowers, in cacti such as prickly pears or the red dragonfruit. It is a powerful antioxidant.

Anthocyanins are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise “Die Farben der Blüthen”. Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.
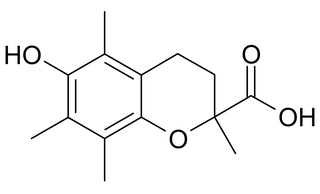
Trolox is a water-soluble analog of vitamin E sold by Hoffman-LaRoche. It is an antioxidant like vitamin E and it is used in biological or biochemical applications to reduce oxidative stress or damage.

Dichlorofluorescein (DCF) is an organic dye of the fluorescein family, being substituted at the 2 and 7 positions by chloride.
TEAC may refer to:

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Ferric reducing ability of plasma is an antioxidant capacity assay that uses Trolox as a standard. The FRAP assay was first performed by Iris Benzie and J. J. Strain of the Human Nutrition Research Group at the University of Ulster, Coleraine. The method is based on the formation of O-Phenanthroline-Fe(2+) complex and its disruption in the presence of chelating agents. This assay is often used to measure the antioxidant capacity of foods, beverages and nutritional supplements containing polyphenols.
Antioxidative stress is an overabundance of bioavailable antioxidant compounds that interfere with the immune system's ability to neutralize pathogenic threats. The fundamental opposite is oxidative stress, which can lead to such disease states as coronary heart disease or cancer.
Selectivity factor is a quantifiable measure of how efficient an antibiotic is during the process of gene selection. It measures of the capacity an antibiotic to select for transfected (resistant) cells that contain a selectable marker, while killing untransfected (sensitive) cells that do not contain a selectable marker. A selectivity factor higher than 10 is optimal. This means the concentration of antibiotic is sufficient to kill untransfected cells but not toxic enough to kill transfected cells. A selectivity factor lower than 10 means the concentration of antibiotic needed for selection is too close to the toxic concentration for the transfected cells. As a result, fewer transfected cells survive and more untransfected cells survive. In this case an alternative antibiotic should be considered.
References
- ↑ Huang, D.; Ou, B.; Prior, R. L. (2005). "The Chemistry behind Antioxidant Capacity Assays". J. Agric. Food Chem. 53 (6): 1841–56. doi:10.1021/jf030723c. PMID 15769103.