Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane.

Red blood cells (RBCs), also referred to as red cells, red blood corpuscles, haematids, erythroid cells or erythrocytes (from Greek erythros for "red" and kytos for "hollow vessel", with -cyte translated as "cell" in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or gills of fish, and release it into tissues while squeezing through the body's capillaries.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in disease, called malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses disease at a microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans, causing 405,000 deaths in 2018. It is also associated with the development of blood cancer and is classified as Group 2A carcinogen.
Glycophorin C plays a functionally important role in maintaining erythrocyte shape and regulating membrane material properties, possibly through its interaction with protein 4.1. Moreover, it has previously been shown that membranes deficient in protein 4.1 exhibit decreased content of glycophorin C. It is also an integral membrane protein of the erythrocyte and acts as the receptor for the Plasmodium falciparum protein PfEBP-2.

Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 is a protein that in humans is encoded by the CR1 gene.
CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.
Silver staining is the use of silver to selectively alter the appearance of a target in microscopy of histological sections; in temperature gradient gel electrophoresis; and in polyacrylamide gels.

Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red blood cell "ghost" (spectre), and so the major protein of the ghost was named spectrin.

Hereditary elliptocytosis, also known as ovalocytosis, is an inherited blood disorder in which an abnormally large number of the person's red blood cells are elliptical rather than the typical biconcave disc shape. Such morphologically distinctive erythrocytes are sometimes referred to as elliptocytes or ovalocytes. It is one of many red-cell membrane defects. In its severe forms, this disorder predisposes to haemolytic anaemia. Although pathological in humans, elliptocytosis is normal in camelids.
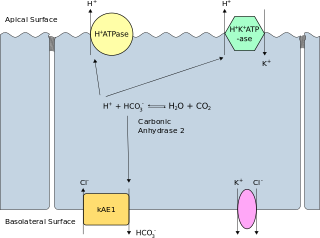
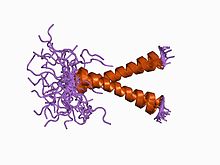
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.

The bafilomycins are a family of macrolide antibiotics produced from a variety of Streptomycetes. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.
The MNS antigen system is a human blood group system based upon two genes on chromosome 4. There are currently 46 antigens in the system, but the five most important are called M, N, S, s, and U.

Glycophorin A , also known as GYPA, is a protein which in humans is encoded by the GYPA gene. GYPA has also recently been designated CD235a.

Glycophorin B also known as sialoglycoprotein delta and SS-active sialoglycoprotein is a protein which in humans is encoded by the GYPB gene. GYPB has also recently been designated CD235b.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most commonly alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.