
A monoclonal antibody is an antibody made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Chimeric antigen receptor T cells are T cells that have been genetically engineered to produce an artificial T cell receptor for use in immunotherapy.
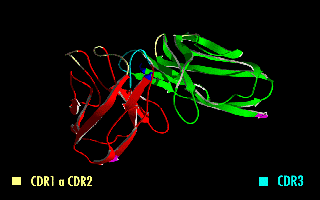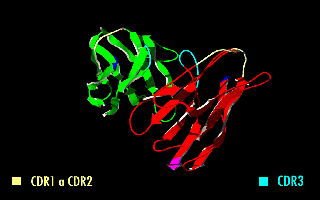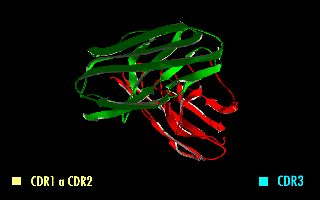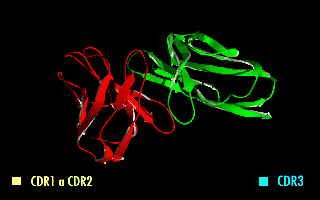
A single-chain variable fragment (scFv) is not actually a fragment of an antibody, but instead is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or vice versa. This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker. The image to the right shows how this modification usually leaves the specificity unaltered.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspeciality of oncology.
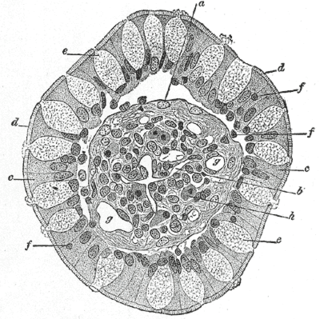
Goblet cells are simple columnar epithelial cells that secrete gel-forming mucins, like mucin MUC5AC. The goblet cells mainly use the merocrine method of secretion, secreting vesicles into a duct, but may use apocrine methods, budding off their secretions, when under stress. The term goblet refers to the cell's goblet-like shape. The apical portion is shaped like a cup, as it is distended by abundant mucus laden granules; its basal portion lacks these granules and is shaped like a stem.

A single-domain antibody (sdAb), also known as a nanobody, is an antibody fragment consisting of a single monomeric variable antibody domain. Like a whole antibody, it is able to bind selectively to a specific antigen. With a molecular weight of only 12–15 kDa, single-domain antibodies are much smaller than common antibodies which are composed of two heavy protein chains and two light chains, and even smaller than Fab fragments and single-chain variable fragments.

The selectins are a family of cell adhesion molecules. All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers.

The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene on chromosome 11. CD44 has been referred to as HCAM, Pgp-1, Hermes antigen, lymphocyte homing receptor, ECM-III, and HUTCH-1.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

Mesothelin, also known as MSLN, is a protein that in humans is encoded by the MSLN gene.

Mucin 1, cell surface associated (MUC1), also called polymorphic epithelial mucin (PEM) or epithelial membrane antigen or EMA, is a mucin encoded by the MUC1 gene in humans. MUC1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Mucins line the apical surface of epithelial cells in the lungs, stomach, intestines, eyes and several other organs. Mucins protect the body from infection by pathogen binding to oligosaccharides in the extracellular domain, preventing the pathogen from reaching the cell surface. Overexpression of MUC1 is often associated with colon, breast, ovarian, lung and pancreatic cancers. Joyce Taylor-Papadimitriou identified and characterised the antigen during her work with breast and ovarian tumors.
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen. Naturally occurring antibodies typically only target one antigen. Upon development, BsAbs can be manufactured in several structural formats. Through different mechanism of action, BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes. BsAbs have advantages compared to ordinary monoclonal antibodies, while BsAbs have problems and disadvantages. The major current applications of BsAbs have been explored for cancer immunotherapy and drug delivery, while BsAbs can also be applied to treat other diseases, including Alzeimer's disease and so on.

CD47 also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin-1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a don't eat me signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis.

Epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein mediating Ca2+-independent homotypic cell–cell adhesion in epithelia. EpCAM is also involved in cell signaling, migration, proliferation, and differentiation. Additionally, EpCAM has oncogenic potential via its capacity to upregulate c-myc, e-fabp, and cyclins A & E. Since EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms, EpCAM can be used as diagnostic marker for various cancers. It appears to play a role in tumorigenesis and metastasis of carcinomas, so it can also act as a potential prognostic marker and as a potential target for immunotherapeutic strategies.

Hepatitis A virus cellular receptor 2 (HAVCR2), also known as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), is a protein that in humans is encoded by the HAVCR2 (TIM-3)gene. HAVCR2 was first described in 2002 as a cell surface molecule expressed on IFNγ producing CD4+ Th1 and CD8+ Tc1 cells. Later, the expression was detected in Th17 cells, regulatory T-cells, and innate immune cells. HAVCR2 receptor is a regulator of the immune response.
Tumor-associated glycoprotein 72 (TAG-72) is a glycoprotein found on the surface of many cancer cells, including ovary, breast, colon, lung, and pancreatic cancers. It is a mucin-like molecule with a molar mass of over 1000 kDa.
A431 cells are a model human cell line used in biomedical research.
A rabbit hybridoma is a hybrid cell line formed by the fusion of an antibody producing rabbit B cell with a cancerous B-cell (myeloma).
Joyce Taylor-Papadimitriou FMedSci is a British molecular biologist and geneticist. She is Senior Fellow and Visiting Professor at King's College London specialising in the area of cellular, genetic and proteomic studies on patient breast tumour samples, and works within the Breast Cancer Biology Group. She was the first to identify that the action of interferon type 1 requires the synthesis of effector proteins.
SK-OV-3 is an ovarian cancer cell line derived from the ascites of a 64-year-old Caucasian female with an ovarian serous cystadenocarcinoma. The SK-OV-3 cell line is also hypodiploid, with a modal number of chromosomes of 43, occurring in 63.3% of cells. SK-OV-3 are positive for many of the antigens used to identify cancers of epithelial origin in clinical practice, including vimentin (VIM), high molecular weight cytokeratin (HMWK), low molecular weight cytokeratin (LMWK), epithelial membrane antigen (EMA) and leucocyte common antigen (LCA).
















