A tendon or sinew is a tough band of dense fibrous connective tissue that connects muscle to bone. It sends the mechanical forces of muscle contraction to the skeletal system, while withstanding tension.

Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge. The Ser residue is generally in the sequence -Ser-Gly-X-Gly-, although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue.

Chondrocytes are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes can differentiate into various cell types, including osteoblasts.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.
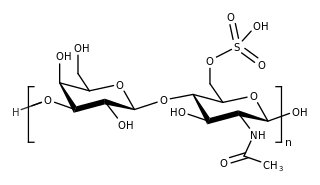
Keratan sulfate (KS), also called keratosulfate, is any of several sulfated glycosaminoglycans that have been found especially in the cornea, cartilage, and bone. It is also synthesized in the central nervous system where it participates both in development and in the glial scar formation following an injury. Keratan sulfates are large, highly hydrated molecules which in joints can act as a cushion to absorb mechanical shock.
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. The name perlecan comes from its appearance as a "string of pearls" in rotary shadowed images.

The stroma of the cornea is a fibrous, tough, unyielding, perfectly transparent and the thickest layer of the cornea of the eye. It is between Bowman's layer anteriorly, and Descemet's membrane posteriorly.

Decorin is a protein that in humans is encoded by the DCN gene.

Bone morphogenetic protein 3, also known as osteogenin, is a protein in humans that is encoded by the BMP3 gene.

Aggrecan (ACAN), also known as cartilage-specific proteoglycan core protein (CSPCP) or chondroitin sulfate proteoglycan 1, is a protein that in humans is encoded by the ACAN gene. This gene is a member of the lectican (chondroitin sulfate proteoglycan) family. The encoded protein is an integral part of the extracellular matrix in cartilagenous tissue and it withstands compression in cartilage.

Collagen alpha-1(V) chain is a protein that in humans is encoded by the COL5A1 gene.

Collagen alpha-6(IV) chain is a protein that in humans is encoded by the COL4A6 gene.

Collagen alpha-2(VI) chain is a protein that in humans is encoded by the COL6A2 gene.

Lumican, also known as LUM, is an extracellular matrix protein that, in humans, is encoded by the LUM gene on chromosome 12.

Fibromodulin is a protein that in humans is encoded by the FMOD gene.

Prolargin is a protein that in humans is encoded by the PRELP gene.

Beta-1,4-galactosyltransferase 7 also known as galactosyltransferase I is an enzyme that in humans is encoded by the B4GALT7 gene. Galactosyltransferase I catalyzes the synthesis of the glycosaminoglycan-protein linkage in proteoglycans. Proteoglycans in turn are structural components of the extracellular matrix that is found between cells in connective tissues.

Collagen alpha-1(XXVI) chain is a protein that in humans is encoded by the EMID2 gene.

Tsukushi or tsukushin is an extracellular matrix protein of the small leucine rich proteoglycan (SLRP) family. In humans it is encoded by the TSKU gene.
Dick Heinegård was a Swedish biochemist. He received his doctorate in 1974 at Lund University and later became a professor of medical and physiological chemistry there. His research concentrated on the biology and pathology of connective tissue. Heinegård was elected in 2002 as a member of the Kungliga Vetenskapsakademin.