
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name biotin, borrowed from the German Biotin, derives from the Ancient Greek word βίοτος (bíotos; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white crystalline solid that looks like needles.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.

Streptavidin is a 52 kDa protein (tetramer) purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin. With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants, detergents, proteolytic enzymes, and extremes of temperature and pH.
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits, and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits, or two heterodimer subunits.
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Tags are attached to proteins for various purposes. They can be added to either end of the target protein, so they are either C-terminus or N-terminus specific or are both C-terminus and N-terminus specific. Some tags are also inserted at sites within the protein of interest; they are known as internal tags.

An electrophoretic mobility shift assay (EMSA) or mobility shift electrophoresis, also referred as a gel shift assay, gel mobility shift assay, band shift assay, or gel retardation assay, is a common affinity electrophoresis technique used to study protein–DNA or protein–RNA interactions. This procedure can determine if a protein or mixture of proteins is capable of binding to a given DNA or RNA sequence, and can sometimes indicate if more than one protein molecule is involved in the binding complex. Gel shift assays are often performed in vitro concurrently with DNase footprinting, primer extension, and promoter-probe experiments when studying transcription initiation, DNA gang replication, DNA repair or RNA processing and maturation, as well as pre-mRNA splicing. Although precursors can be found in earlier literature, most current assays are based on methods described by Garner and Revzin and Fried and Crothers.
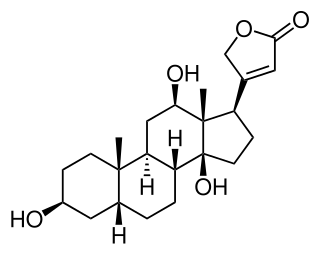
Digoxigenin (DIG) is a steroid found exclusively in the flowers and leaves of the plants Digitalis purpurea, Digitalis orientalis and Digitalis lanata (foxgloves), where it is attached to sugars, to form the glycosides.
A tetramer assay is a procedure that uses tetrameric proteins to detect and quantify T cells that are specific for a given antigen within a blood sample. The tetramers used in the assay are made up of four major histocompatibility complex (MHC) molecules, which are found on the surface of most cells in the body. MHC molecules present peptides to T-cells as a way to communicate the presence of viruses, bacteria, cancerous mutations, or other antigens in a cell. If a T-cell's receptor matches the peptide being presented by an MHC molecule, an immune response is triggered. Thus, MHC tetramers that are bioengineered to present a specific peptide can be used to find T-cells with receptors that match that peptide. The tetramers are labeled with a fluorophore, allowing tetramer-bound T-cells to be analyzed with flow cytometry. Quantification and sorting of T-cells by flow cytometry enables researchers to investigate immune response to viral infection and vaccine administration as well as functionality of antigen-specific T-cells. Generally, if a person's immune system has encountered a pathogen, the individual will possess T cells with specificity toward some peptide on that pathogen. Hence, if a tetramer stain specific for a pathogenic peptide results in a positive signal, this may indicate that the person's immune system has encountered and built a response to that pathogen.
NeutrAvidin protein is a deglycosylated version of chicken avidin, with a mass of approximately 60,000 daltons. As a result of carbohydrate removal, lectin binding is reduced to undetectable levels, yet biotin binding affinity is retained because the carbohydrate is not necessary for this activity. Avidin has a high pI but NeutrAvidin has a near-neutral pI, minimizing non-specific interactions with the negatively-charged cell surface or with DNA/RNA. Neutravidin still has lysine residues that remain available for derivatization or conjugation.

Biotin deficiency is a nutritional disorder which can become serious, even fatal, if allowed to progress untreated. It can occur in people of any age, ancestry, or of either sex. Biotin is part of the B vitamin family. Biotin deficiency rarely occurs among healthy people because the daily requirement of biotin is low, many foods provide adequate amounts of it, intestinal bacteria synthesize small amounts of it, and the body effectively scavenges and recycles it in the kidneys during production of urine.
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding.

Meir Wilchek is an Israeli biochemist. He is a professor at the Weizmann Institute of Science.
The Strep-tag system is a method which allows the purification and detection of proteins by affinity chromatography. The Strep-tag II is a synthetic peptide consisting of eight amino acids (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys). This peptide sequence exhibits intrinsic affinity towards Strep-Tactin, a specifically engineered streptavidin, and can be N- or C- terminally fused to recombinant proteins. By exploiting the highly specific interaction, Strep-tagged proteins can be isolated in one step from crude cell lysates. Because the Strep-tag elutes under gentle, physiological conditions, it is especially suited for generation of functional proteins.
The Streptavidin-Binding Peptide (SBP)-Tag is a 38-amino acid sequence that may be engineered into recombinant proteins. Recombinant proteins containing the SBP-Tag bind to streptavidin and this property may be utilized in specific purification, detection or immobilization strategies.

Paul György (April 7, 1893 – March 1, 1976) was a Hungarian-born American biochemist, nutritionist, and pediatrician best known for his discovery of three B vitamins: riboflavin, B6, and biotin. Gyorgy was also well known for his research into the protective factors of human breast milk, particularly for his discoveries of Lactobacillus bifidus growth factor activity in human milk and its anti-staphylococcal properties. He was a recipient of the National Medal of Science in 1975 from President Gerald Ford.
MHC multimers are oligomeric forms of MHC molecules, designed to identify and isolate T-cells with high affinity to specific antigens amid a large group of unrelated T-cells. Multimers generally range in size from dimers to octamers; however, some companies use even higher quantities of MHC per multimer. Multimers may be used to display class 1 MHC, class 2 MHC, or nonclassical molecules from species such as monkeys, mice, and humans.
Chem-seq is a technique that is used to map genome-wide interactions between small molecules and their protein targets in the chromatin of eukaryotic cell nuclei. The method employs chemical affinity capture coupled with massively parallel DNA sequencing to identify genomic sites where small molecules interact with their target proteins or DNA. It was first described by Lars Anders et al. in the January, 2014 issue of "Nature Biotechnology".
Esmond Emerson Snell (September 22, 1914 – December 9, 2003) was an American biochemist who spent his career researching vitamins and nutritional requirements of bacteria and yeast. He is well known for his study of lactic acid-producing bacteria, developing microbiological assays for a number of key nutrients; the discovery of more than half of known vitamins has been attributed to the use of this work. He discovered several B vitamins, including folic acid, and characterized the biochemistry of vitamin B6 (also known as pyrixodal).

IBA Lifesciences is a biotechnology company providing products and custom specific services for life science applications in academia and industry worldwide. IBA focusses on two business segments: cell selection and protein purification.
BLESS, also known as breaks labeling, enrichment on streptavidin and next-generation sequencing, is a method used to detect genome-wide double-strand DNA damage. In contrast to chromatin immunoprecipitation (ChIP)-based methods of identifying DNA double-strand breaks (DSBs) by labeling DNA repair proteins, BLESS utilizes biotinylated DNA linkers to directly label genomic DNA in situ which allows for high-specificity enrichment of samples on streptavidin beads and the subsequent sequencing-based DSB mapping to nucleotide resolution.









