Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Interneurons are neurons that connect to brain regions, i.e. not direct motor neurons or sensory neurons. Interneurons are the central nodes of neural circuits, enabling communication between sensory or motor neurons and the central nervous system (CNS). They play vital roles in reflexes, neuronal oscillations, and neurogenesis in the adult mammalian brain.

Sarcoplasm is the cytoplasm of a muscle cell. It is comparable to the cytoplasm of other cells, but it contains unusually large amounts of glycogen (a polymer of glucose), myoglobin, a red-colored protein necessary for binding oxygen molecules that diffuse into muscle fibers, and mitochondria. The calcium ion concentration in sarcoplasma is also a special element of the muscle fiber; it is the means by which muscle contractions take place and are regulated. The sarcoplasm plays a critical role in muscle contraction as an increase in Ca2+ concentration in the sarcoplasm begins the process of filament sliding. The decrease in Ca2+ in the sarcoplasm subsequently ceases filament sliding. The sarcoplasm also aids in pH and ion balance within muscle cells.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g. muscle, glial cells, neurons) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.

Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in many animal and fungal cells. In animals, it is an important component of the muscular system which works in conjunction with troponin to regulate muscle contraction. It is present in smooth and striated muscle tissues, which can be found in various organs and body systems, including the heart, blood vessels, respiratory system, and digestive system. In fungi, tropomyosin is found in cell walls and helps maintain the structural integrity of cells.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
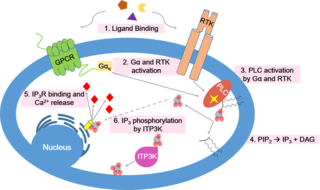
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.

Calbindins are three different calcium-binding proteins: calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney of chicks and mammals. They are now classified in different subfamilies as they differ in the number of Ca2+ binding EF hands.
Oncomodulin is a parvalbumin-family calcium-binding protein expressed and secreted by macrophages.
Calcium-binding proteins are proteins that participate in calcium cell signaling pathways by binding to Ca2+, the calcium ion that plays an important role in many cellular processes. Calcium-binding proteins have specific domains that bind to calcium and are known to be heterogeneous.

Calretinin, also known as calbindin 2, is a calcium-binding protein involved in calcium signaling. In humans, the calretinin protein is encoded by the CALB2 gene.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound THC which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV).

Protein S100-A1, also known as S100 calcium-binding protein A1 is a protein which in humans is encoded by the S100A1 gene. S100A1 is highly expressed in cardiac and skeletal muscle, and localizes to Z-discs and sarcoplasmic reticulum. S100A1 has shown promise as an effective candidate for gene therapy to treat post-myocardially infarcted cardiac tissue.
S100 calcium-binding protein P (S100P) is a protein that in humans is encoded by the S100P gene.

Stromal interaction molecule 2 (STIM2) is a protein that in humans is encoded by the STIM2 gene.
ParvE101Q is an experimental modification of parvalbumin, designed to delay calcium sequestration in heart muscles to enhance contraction. The protein parvalbumin has EF hand motifs used for calcium binding. EF hands are structural helix-loop-helix protein subunits that have a high affinity for calcium ions, and a moderate affinity for magnesium ions. In muscle, the binding of Ca2+ by parvalbumin efficiently sequesters it following contraction. This increases the speed of muscle relaxation, allowing the muscle to contract again sooner. Although parvalbumin is classified as a delayed calcium buffer, it quickly sequesters Ca2+, usually before the muscle is done fully contracting. Large amounts of parvalbumin allow rapid contractions of muscles at a high contractile speed with the trade-off of having relatively lower contraction force. This decreased force of contraction is due to the rapid sequestration of Ca2+, preventing prolonged contraction which is required for greater force.
Calcium imaging is a microscopy technique to optically measure the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging takes advantage of calcium indicators, fluorescent molecules that respond to the binding of Ca2+ ions by fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI). This technique has allowed studies of calcium signalling in a wide variety of cell types. In neurons, action potential generation is always accompanied by rapid influx of Ca2+ ions. Thus, calcium imaging can be used to monitor the electrical activity in hundreds of neurons in cell culture or in living animals, which has made it possible to observe the activity of neuronal circuits during ongoing behavior.

Fish allergy is an immune hypersensitivity to proteins found in fish. Symptoms can be either rapid or gradual in onset. The latter can take hours to days to appear. The former may include anaphylaxis, a potentially life-threatening condition which requires treatment with epinephrine. Other presentations may include atopic dermatitis or inflammation of the esophagus. Fish is one of the eight common food allergens which are responsible for 90% of allergic reactions to foods: cow's milk, eggs, wheat, shellfish, peanuts, tree nuts, fish, and soy beans.




















