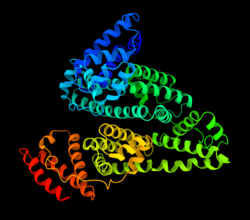
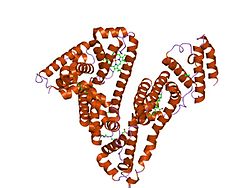
Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.[ citation needed ]
Albumin transports hormones, fatty acids, and other compounds, buffers pH, and maintains oncotic pressure, among other functions.
Albumin is synthesized in the liver as preproalbumin, which has an N-terminal peptide that is removed before the nascent protein is released from the rough endoplasmic reticulum. The product, proalbumin, is in turn cleaved in the Golgi apparatus to produce the secreted albumin.
The reference range for albumin concentrations in serum is approximately 35–50 g/L (3.5–5.0 g/dL). [5] It has a serum half-life of approximately 21 days. [6] It has a molecular mass of 66.5 kDa.
The gene for albumin is located on chromosome 4 in locus 4q13.3 and mutations in this gene can result in anomalous proteins. The human albumin gene is 16,961 nucleotides long from the putative 'cap' site to the first poly(A) addition site. It is split into 15 exons that are symmetrically placed within the 3 domains thought to have arisen by triplication of a single primordial domain.
Human serum albumin (HSA) is a highly water-soluble globular monomeric plasma protein with a relative molecular weight of 67 KDa, consisting of 585 amino acid residues, one sulfhydryl group and 17 disulfide bridges. Among nanoparticulate carriers, HSA nanoparticles have long been the center of attention in the pharmaceutical industry due to their ability to bind to various drug molecules, great stability during storage and in vivo usage, no toxicity and antigenicity, biodegradability, reproducibility, scale up of the production process and a better control over release properties. In addition, significant amounts of drug can be incorporated into the particle matrix because of the large number of drug binding sites on the albumin molecule. [7]