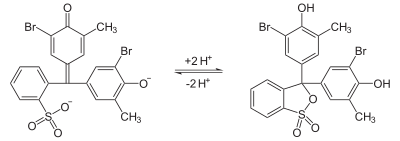
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids.
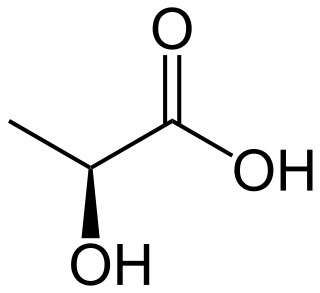
Lactic acid is an organic acid. It has the molecular formula C3H6O3. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl.

Sourdough or sourdough bread is a bread made by allowing the dough to ferment using naturally occurring lactobacillaceae and yeast before baking. The fermentation process produces lactic acid, which gives the bread a sour taste and improves its keeping-qualities.

Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.

Malolactic conversion is a process in winemaking in which tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation is most often performed as a secondary fermentation shortly after the end of the primary fermentation, but can sometimes run concurrently with it. The process is standard for most red wine production and common for some white grape varieties such as Chardonnay, where it can impart a "buttery" flavor from diacetyl, a byproduct of the reaction.
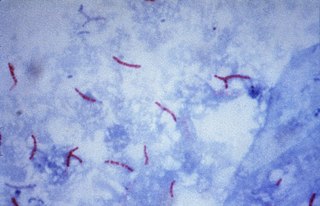
The Ziehl-Neelsen stain, also known as the acid-fast stain, is a bacteriological staining technique used in cytopathology and microbiology to identify acid-fast bacteria under microscopy, particularly members of the Mycobacterium genus. This staining method was initially introduced by Paul Ehrlich (1854–1915) and subsequently modified by the German bacteriologists Franz Ziehl (1859–1926) and Friedrich Neelsen (1854–1898) during the late 19th century.
Fructilactobacillus sanfranciscensis is a heterofermentative species of lactic acid bacteria which, through the production mainly of lactic and acetic acids, helps give sourdough bread its characteristic taste. It is named after San Francisco, where sourdough was found to contain the variety, though it is dominant in Type I sourdoughs globally. In fact, F. sanfranciscensis has been used in sourdough breads for thousands of years, and is used in 3 million tons of sourdough goods yearly. For commercial use, specific strains of F. sanfranciscensis are grown on defined media, freeze-dried, and shipped to bakeries worldwide.
Bovine serum albumin is a serum albumin protein derived from cows. It is often used as a protein concentration standard in lab experiments.

Lactobacillales are an order of gram-positive, low-GC, acid-tolerant, generally nonsporulating, nonrespiring, either rod-shaped (bacilli) or spherical (cocci) bacteria that share common metabolic and physiological characteristics. These bacteria, usually found in decomposing plants and milk products, produce lactic acid as the major metabolic end product of carbohydrate fermentation, giving them the common name lactic acid bacteria (LAB).

Human serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.
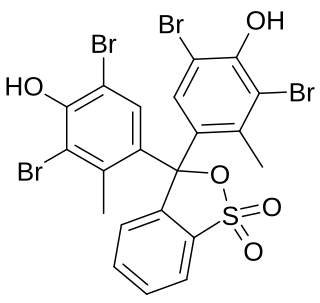
Bromocresol green (BCG) is a dye of the triphenylmethane family. It belongs to a class of dyes called sulfonephthaleins. It is used as a pH indicator in applications such as growth mediums for microorganisms and titrations. In clinical practise, it is commonly used as a diagnostic technique. The most common use of bromocresol green is to measure serum albumin concentration within mammalian blood samples in possible cases of kidney failure and liver disease. In chemistry, bromocresol green is used in Thin-layer chromatography staining solutions to visualize acidic compounds.

Fermentation is a type of redox metabolism carried out in the absence of oxygen. During fermentation, organic molecules are catabolized and donate electrons to other organic molecules. In the process, ATP and organic end products are formed.
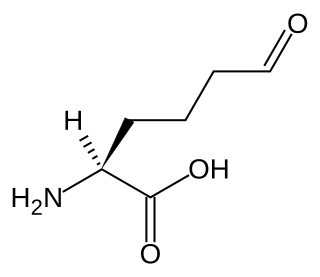
Allysine is a derivative of lysine that features a formyl group in place of the terminal amine. The free amino acid does not exist, but the allysine residue does. It is produced by aerobic oxidation of lysine residues by the enzyme lysyl oxidase. The transformation is an example of a post-translational modification. The semialdehyde form exists in equilibrium with a cyclic derivative.

Trichophyton rubrum is a dermatophytic fungus in the phylum Ascomycota. It is an exclusively clonal, anthropophilic saprotroph that colonizes the upper layers of dead skin, and is the most common cause of athlete's foot, fungal infection of nail, jock itch, and ringworm worldwide. Trichophyton rubrum was first described by Malmsten in 1845 and is currently considered to be a complex of species that comprises multiple, geographically patterned morphotypes, several of which have been formally described as distinct taxa, including T. raubitschekii, T. gourvilii, T. megninii and T. soudanense.

Symbiotic culture of bacteria and yeast (SCOBY) is a culinary symbiotic fermentation culture (starter) consisting of lactic acid bacteria (LAB), acetic acid bacteria (AAB), and yeast which arises in the preparation of sour foods and beverages such as kombucha. Beer and wine also undergo fermentation with yeast, but the lactic acid bacteria and acetic acid bacteria components unique to SCOBY are usually viewed as a source of spoilage rather than a desired addition. Both LAB and AAB enter on the surface of barley and malt in beer fermentation and grapes in wine fermentation; LAB lowers the pH of the beer/wine while AAB takes the ethanol produced from the yeast and oxidizes it further into vinegar, resulting in a sour taste and smell. AAB are also responsible for the formation of the cellulose SCOBY.
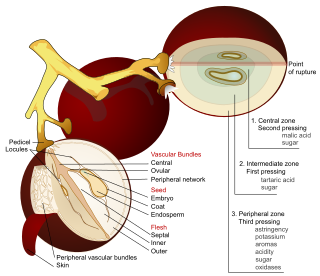
The acids in wine are an important component in both winemaking and the finished product of wine. They are present in both grapes and wine, having direct influences on the color, balance and taste of the wine as well as the growth and vitality of yeast during fermentation and protecting the wine from bacteria. The measure of the amount of acidity in wine is known as the “titratable acidity” or “total acidity”, which refers to the test that yields the total of all acids present, while strength of acidity is measured according to pH, with most wines having a pH between 2.9 and 3.9. Generally, the lower the pH, the higher the acidity in the wine. There is no direct connection between total acidity and pH. In wine tasting, the term “acidity” refers to the fresh, tart and sour attributes of the wine which are evaluated in relation to how well the acidity balances out the sweetness and bitter components of the wine such as tannins. Three primary acids are found in wine grapes: tartaric, malic, and citric acids. During the course of winemaking and in the finished wines, acetic, butyric, lactic, and succinic acids can play significant roles. Most of the acids involved with wine are fixed acids with the notable exception of acetic acid, mostly found in vinegar, which is volatile and can contribute to the wine fault known as volatile acidity. Sometimes, additional acids, such as ascorbic, sorbic and sulfurous acids, are used in winemaking.
Trichophyton tonsurans is a fungus in the family Arthrodermataceae that causes ringworm infection of the scalp. It was first recognized by David Gruby in 1844. Isolates are characterized as the "–" or negative mating type of the Arthroderma vanbreuseghemii complex. This species is thought to be conspecific with T. equinum, although the latter represents the "+" mating strain of the same biological species Despite their biological conspecificity, clones of the two mating types appear to have undergone evolutionary divergence with isolates of the T. tonsurans-type consistently associated with Tinea capitis whereas the T. equinum-type, as its name implies, is associated with horses as a regular host. Phylogenetic relationships were established in isolates from Northern Brazil, through fingerprinting polymorphic RAPD and M13 markers. There seems to be lower genomic variability in the T. tonsurans species due to allopatric divergence. Any phenotypic density is likely due to environmental factors, not genetic characteristics of the fungus.

Biopreservation is the use of natural or controlled microbiota or antimicrobials as a way of preserving food and extending its shelf life. The biopreservation of food, especially utilizing lactic acid bacteria (LAB) that are inhibitory to food spoilage microbes, has been practiced since early ages, at first unconsciously but eventually with an increasingly robust scientific foundation. Beneficial bacteria or the fermentation products produced by these bacteria are used in biopreservation to control spoilage and render pathogens inactive in food. There are a various modes of action through which microorganisms can interfere with the growth of others such as organic acid production, resulting in a reduction of pH and the antimicrobial activity of the un-dissociated acid molecules, a wide variety of small inhibitory molecules including hydrogen peroxide, etc. It is a benign ecological approach which is gaining increasing attention.

Fermented sausage, or dry sausage, is a type of sausage that is created by salting chopped or ground meat to remove moisture, while allowing beneficial bacteria to break down sugars into flavorful molecules. Bacteria, including Lactobacillus species and Leuconostoc species, break down these sugars to produce lactic acid, which not only affects the flavor of the sausage, but also lowers the pH from 6.0 to 4.5–5.0, preventing the growth of bacteria that could spoil the sausage. These effects are magnified during the drying process, as the salt and acidity are concentrated as moisture is extracted.
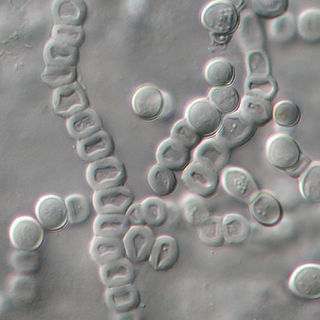
Trichophyton verrucosum, commonly known as the cattle ringworm fungus, is a dermatophyte largely responsible for fungal skin disease in cattle, but is also a common cause of ringworm in donkeys, dogs, goat, sheep, and horses. It has a worldwide distribution, however human infection is more common in rural areas where contact with animals is more frequent, and can cause severe inflammation of the afflicted region. Trichophyton verrucosum was first described by Emile Bodin in 1902.