Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the backbone may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure. A number of these structures may bind to each other, forming a quaternary structure.

Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 464-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (thrombin) and factor Xa.

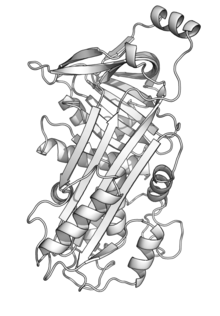
Lysozyme is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside hydrolase that catalyzes the following process:

Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases. They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site.
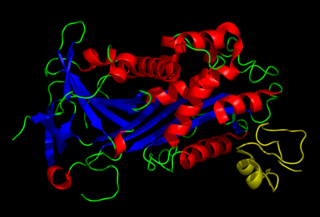
Vitronectin is a glycoprotein of the hemopexin family which is synthesized and excreted by the liver, and abundantly found in serum, the extracellular matrix and bone. In humans it is encoded by the VTN gene.
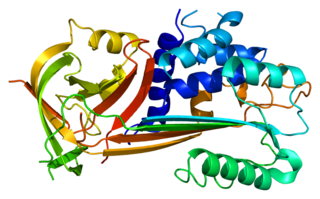
Plasminogen activator inhibitor-2, a serine protease inhibitor of the serpin superfamily, is a coagulation factor that inactivates tissue plasminogen activator and urokinase. It is present in most cells, especially monocytes/macrophages. PAI-2 exists in two forms, a 60-kDa extracellular glycosylated form and a 43-kDa intracellular form.

Ovomucoid is a protein found in egg whites. It is a trypsin inhibitor with three protein domains of the Kazal domain family. The homologs from chickens and especially turkeys are best characterized. It is not related to the similarly named ovomucin, another egg white protein.

Thermolysin is a thermostable neutral metalloproteinase enzyme produced by the Gram-positive bacteria Bacillus thermoproteolyticus. It requires one zinc ion for enzyme activity and four calcium ions for structural stability. Thermolysin specifically catalyzes the hydrolysis of peptide bonds containing hydrophobic amino acids. However thermolysin is also widely used for peptide bond formation through the reverse reaction of hydrolysis. Thermolysin is the most stable member of a family of metalloproteinases produced by various Bacillus species. These enzymes are also termed 'neutral' proteinases or thermolysin -like proteinases (TLPs).

Protein C inhibitor is a serine protease inhibitor (serpin) that limits the activity of protein C.
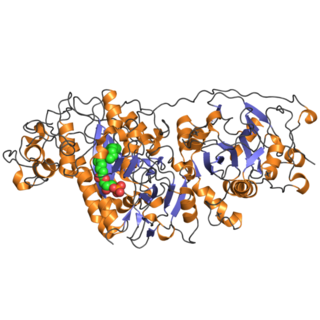
Autotaxin, also known as ectonucleotide pyrophosphatase/phosphodiesterase family member 2, is an enzyme that in humans is encoded by the ENPP2 gene.

Maspin is a protein that in humans is encoded by the SERPINB5 gene. This protein belongs to the serpin superfamily. SERPINB5 was originally reported to function as a tumor suppressor gene in epithelial cells, suppressing the ability of cancer cells to invade and metastasize to other tissues. Furthermore, and consistent with an important biological function, Maspin knockout mice were reported to be non-viable, dying in early embryogenesis. However, a subsequent study using viral transduction as a method of gene transfer was not able to reproduce the original findings and found no role for maspin in tumour biology. Furthermore, the latter study demonstrated that maspin knockout mice are viable and display no obvious phenotype. These data are consistent with the observation that maspin is not expressed in early embryogenesis. The precise molecular function of maspin is thus currently unknown.

Proteinase-activated receptor 1 (PAR1) also known as protease-activated receptor 1, coagulation factor II receptor and thrombin receptor is a protein that in humans is encoded by the F2R gene. PAR1 is a G protein-coupled receptor and one of four protease-activated receptors involved in the regulation of thrombotic response. Highly expressed in platelets and endothelial cells, PAR1 plays a key role in mediating the interplay between coagulation and inflammation, which is important in the pathogenesis of inflammatory and fibrotic lung diseases. It is also involved both in disruption and maintenance of endothelial barrier integrity, through interaction with either thrombin or activated protein C, respectively.

Serpin B6 is a protein that in humans is encoded by the SERPINB6 gene.

Leukocyte elastase inhibitor (LEI) also known as serpin B1 is a protein that in humans is encoded by the SERPINB1 gene. It is a member of the clade B serpins or ov-serpins founded by ovalbumin.

Sentrin-specific protease 1 is an enzyme that in humans is encoded by the SENP1 gene.

Serpin B8 is a protein that in humans is encoded by the SERPINB8 gene.
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred. Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent. Superfamilies typically contain several protein families which show sequence similarity within each family. The term protein clan is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems.

Serpin peptidase inhibitor, clade B (ovalbumin), member 10 is a protein that in humans is encoded by the SERPINB10 gene.
Colin Llewellyn Raston is a Professor of Chemistry of Flinders University in Adelaide, South Australia and the Premier's Professorial Fellow in Clean Technology. In 2015, he was awarded an Ig Nobel Prize in "for inventing a chemical recipe to partially un-boil an egg". In 2016, Raston was made an Officer of the Order of Australia for his services to science.