
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.

Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases. They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site.
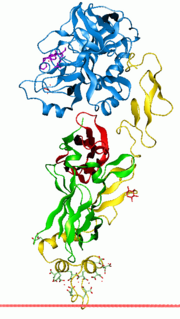
Factor VII is one of the proteins that causes blood to clot in the coagulation cascade. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa, which in turn activates factor IX and factor X.

Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.
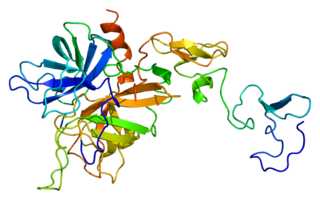
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIX, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.
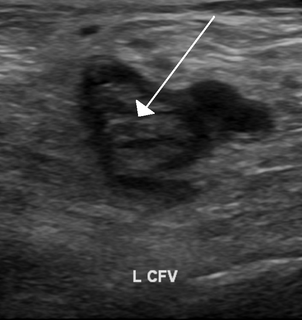
Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.

Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.

Protein Z is a protein which in humans is encoded by the PROZ gene.
Protease-activated receptors (PAR) are a subfamily of related G protein-coupled receptors that are activated by cleavage of part of their extracellular domain. They are highly expressed in platelets, and also on endothelial cells, myocytes and neurons.

Eosinophil major basic protein, often shortened to major basic protein is encoded in humans by the PRG2 gene.
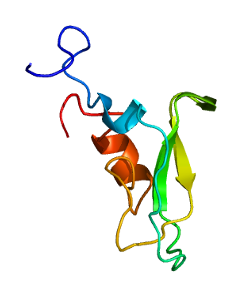
Tissue factor pathway inhibitor is a single-chain polypeptide which can reversibly inhibit Factor Xa (Xa). While Xa is inhibited, the Xa-TFPI complex can subsequently also inhibit the FVIIa-tissue factor complex. TFPI contributes significantly to the inhibition of Xa in vivo, despite being present at concentrations of only 2.5 nM.

Protein C inhibitor is a serine protease inhibitor (serpin) that limits the activity of protein C.

Baculoviral IAP repeat-containing protein3 is a protein that in humans is encoded by the BIRC3 gene.

Transcription factor E2F4 is a protein that in humans is encoded by the E2F4 gene.

Forkhead box O3, also known as FOXO3 or FOXO3a, is a human protein encoded by the FOXO3 gene.

Protease-activated receptor 4 (PAR-4), also known as coagulation factor II (thrombin) receptor-like 3, is a protein that in humans is encoded by the F2RL3 gene.

Calpastatin is a protein that in humans is encoded by the CAST gene.

Serpin B6 is a protein that in humans is encoded by the SERPINB6 gene.



















