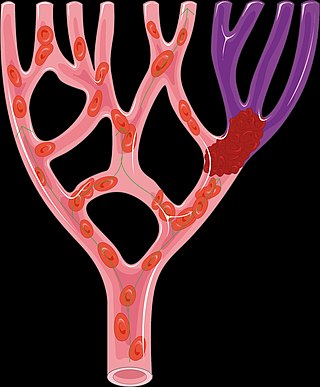
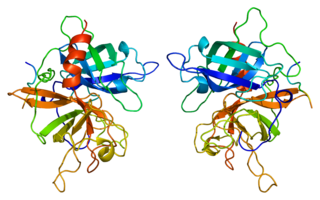A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to stop and prevent further bleeding, but can be harmful in thrombosis, when a clot obstructs blood flow through a healthy blood vessel in the circulatory system.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.
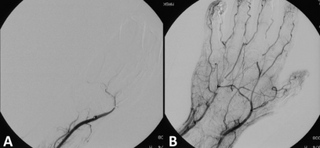
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.
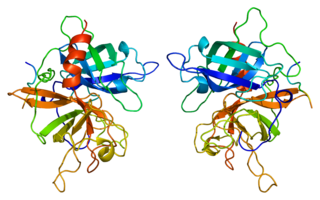
Tissue-type plasminogen activator, short name tPA, is a protein that facilitates the breakdown of blood clots. It acts as an enzyme to convert plasminogen into its active form plasmin, the major enzyme responsible for clot breakdown. It is a serine protease found on endothelial cells lining the blood vessels. Human tPA is encoded by the PLAT gene, and has a molecular weight of ~70 kDa in the single-chain form.

Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.
D-dimer is a dimer that is a fibrin degradation product (FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link, hence forming a protein dimer.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.
Alteplase, sold under the brand name Activase among others, is a biosynthetic form of human tissue-type plasminogen activator (t-PA). It is a thrombolytic medication used to treat acute ischemic stroke, acute ST-elevation myocardial infarction, pulmonary embolism associated with low blood pressure, and blocked central venous catheter. Alteplase is given by injection into a vein or artery. Alteplase is the same as the normal human plasminogen activator produced in vascular endothelial cells and is synthesized via recombinant DNA technology in Chinese hamster ovary cells (CHO). Alteplase causes the breakdown of a clot by inducing fibrinolysis.

Alpha 2-antiplasmin is a serine protease inhibitor (serpin) responsible for inactivating plasmin. Plasmin is an important enzyme that participates in fibrinolysis and degradation of various other proteins. This protein is encoded by the SERPINF2 gene.
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.

Aminocaproic acid is a derivative and analogue of the amino acid lysine, which makes it an effective inhibitor for enzymes that bind that particular residue. Such enzymes include proteolytic enzymes like plasmin, the enzyme responsible for fibrinolysis. For this reason it is effective in treatment of certain bleeding disorders, and it is sold under the brand name Amicar. Aminocaproic acid is also an intermediate in the polymerization of Nylon-6, where it is formed by ring-opening hydrolysis of caprolactam. The crystal structure determination showed that the 6-aminohexanoic acid is present as a salt, at least in the solid state.
Antifibrinolytics are a class of medication that are inhibitors of fibrinolysis. Examples include aminocaproic acid and tranexamic acid. These lysine-like drugs interfere with the formation of the fibrinolytic enzyme plasmin from its precursor plasminogen by plasminogen activators which takes place mainly in lysine rich areas on the surface of fibrin.
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.

Quebec platelet disorder (QPD) is a rare autosomal dominant bleeding disorder first described in a family from the province of Quebec, Canada. The disorder is characterized by large amounts of the fibrinolytic enzyme urokinase-type plasminogen activator (uPA) in platelets. This causes accelerated fibrinolysis which can result in bleeding.
The fibrinolysis system is responsible for removing blood clots. Hyperfibrinolysis describes a situation with markedly enhanced fibrinolytic activity, resulting in increased, sometimes catastrophic bleeding. Hyperfibrinolysis can be caused by acquired or congenital reasons. Among the congenital conditions for hyperfibrinolysis, alpha-2-plasmin inhibitor deficiency or plasminogen activator inhibitor type 1 (PAI-1) are very rare. The affected individuals show a hemophilia-like bleeding phenotype. Acquired hyperfibrinolysis is found in liver disease, in patients with severe trauma, during major surgical procedures, and other conditions. A special situation with temporarily enhanced fibrinolysis is thrombolytic therapy with drugs which activate plasminogen, e.g. for use in acute ischemic events or in patients with stroke. In patients with severe trauma, hyperfibrinolysis is associated with poor outcome. Moreover, hyperfibrinolysis may be associated with blood brain barrier impairment, a plasmin-dependent effect due to an increased generation of bradykinin.
Plasmin-α2-antiplasmin complex (PAP) is a 1:1 irreversibly formed inactive complex of the enzyme plasmin and its inhibitor α2-antiplasmin. It is a marker of the activity of the fibrinolytic system and a marker of net activation of fibrinolysis.
Alpha-2-plasmin inhibitor deficiency, also known as alpha-2-antiplasmindeficiency or congenital alpha-2-antiplasmin deficiency, is a rare autosomal recessive coagulopathy characterized by impaired inhibition of plasmin, leading to increased fibrinolysis and a heightened risk of bleeding.