Related Research Articles

A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to stop and prevent further bleeding, but can be harmful in thrombosis, when a clot obstructs blood flow through a healthy blood vessel in the circulatory system.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.

Fibrinogen is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.
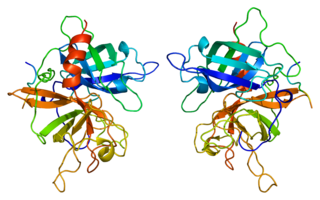
Tissue-type plasminogen activator, short name tPA, is a protein that facilitates the breakdown of blood clots. It acts as an enzyme to convert plasminogen into its active form plasmin, the major enzyme responsible for clot breakdown. It is a serine protease found on endothelial cells lining the blood vessels. Human tPA is encoded by the PLAT gene, and has a molecular weight of ~70 kDa in the single-chain form.

Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.
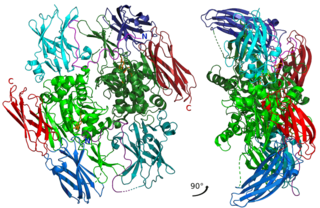
Factor XIII, or fibrin stabilizing factor, is a plasma protein and zymogen. It is activated by thrombin to factor XIIIa which crosslinks fibrin in coagulation. Deficiency of XIII worsens clot stability and increases bleeding tendency.

Alpha 2-antiplasmin is a serine protease inhibitor (serpin) responsible for inactivating plasmin. Plasmin is an important enzyme that participates in fibrinolysis and degradation of various other proteins. This protein is encoded by the SERPINF2 gene.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.

Aminocaproic acid is a derivative and analogue of the amino acid lysine, which makes it an effective inhibitor for enzymes that bind that particular residue. Such enzymes include proteolytic enzymes like plasmin, the enzyme responsible for fibrinolysis. For this reason it is effective in treatment of certain bleeding disorders, and it is sold under the brand name Amicar. Aminocaproic acid is also an intermediate in the polymerization of Nylon-6, where it is formed by ring-opening hydrolysis of caprolactam. The crystal structure determination showed that the 6-aminohexanoic acid is present as a salt, at least in the solid state.
Thromboelastography (TEG) is a method of testing the efficiency of blood coagulation. It is a test mainly used in surgery and anesthesiology, although increasingly used in resuscitations in emergency departments, intensive care units, and labor and delivery suites. More common tests of blood coagulation include prothrombin time (PT) and partial thromboplastin time (aPTT) which measure coagulation factor function, but TEG also can assess platelet function, clot strength, and fibrinolysis which these other tests cannot.
Antifibrinolytics are a class of medication that are inhibitors of fibrinolysis. Examples include aminocaproic acid and tranexamic acid. These lysine-like drugs interfere with the formation of the fibrinolytic enzyme plasmin from its precursor plasminogen by plasminogen activators which takes place mainly in lysine rich areas on the surface of fibrin.
The dysfibrinogenemias consist of three types of fibrinogen disorders in which a critical blood clotting factor, fibrinogen, circulates at normal levels but is dysfunctional. Congenital dysfibrinogenemia is an inherited disorder in which one of the parental genes produces an abnormal fibrinogen. This fibrinogen interferes with normal blood clotting and/or lysis of blood clots. The condition therefore may cause pathological bleeding and/or thrombosis. Acquired dysfibrinogenemia is a non-hereditary disorder in which fibrinogen is dysfunctional due to the presence of liver disease, autoimmune disease, a plasma cell dyscrasias, or certain cancers. It is associated primarily with pathological bleeding. Hereditary fibrinogen Aα-Chain amyloidosis is a sub-category of congenital dysfibrinogenemia in which the dysfunctional fibrinogen does not cause bleeding or thrombosis but rather gradually accumulates in, and disrupts the function of, the kidney.

Quebec platelet disorder (QPD) is a rare autosomal dominant bleeding disorder first described in a family from the province of Quebec, Canada. The disorder is characterized by large amounts of the fibrinolytic enzyme urokinase-type plasminogen activator (uPA) in platelets. This causes accelerated fibrinolysis which can result in bleeding.

Fibrin glue is a surgical formulation used to create a fibrin clot for hemostasis, cartilage repair surgeries or wound healing. It contains separately packaged human fibrinogen and human thrombin.
Thromboelastometry (TEM), previously named rotational thromboelastography (ROTEG) or rotational thromboelastometry (ROTEM), is an established viscoelastic method for hemostasis testing in whole blood. It is a modification of traditional thromboelastography (TEG).
Plasmin-α2-antiplasmin complex (PAP) is a 1:1 irreversibly formed inactive complex of the enzyme plasmin and its inhibitor α2-antiplasmin. It is a marker of the activity of the fibrinolytic system and a marker of net activation of fibrinolysis.
Alpha-2-plasmin inhibitor deficiency, also known as alpha-2-antiplasmindeficiency or congenital alpha-2-antiplasmin deficiency, is a rare autosomal recessive coagulopathy characterized by impaired inhibition of plasmin, leading to increased fibrinolysis and a heightened risk of bleeding.
References
- ↑ Carpenter SL, Mathew P (2008). "Alpha-2-antiplasmin and its deficiency: fibrinolysis out of balance". Haemophilia. 14 (6): 1250–4. doi: 10.1111/j.1365-2516.2008.01766.x . PMID 19141165.
- ↑ Takahashi Y, Tanaka T, Minowa H, Ookubo Y, Sugimoto M, Nakajima M, Miyauchi Y, Yoshioka A (July 1996). "Hereditary partial deficiency of plasminogen activator inhibitor-1 associated with a lifelong bleeding tendency". International Journal of Hematology. 64 (1): 61–8. doi:10.1016/0925-5710(96)00460-4. PMID 8757969.
- ↑ Görlinger K (August 2006). "[Coagulation management during liver transplantation]". Hamostaseologie (in German). 26 (3 Suppl 1): S64–76. doi:10.1055/s-0037-1617084. PMID 16953295.
- ↑ Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, David JS (2008). "Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients". Br J Anaesth. 100 (6): 792–7. doi: 10.1093/bja/aen083 . PMID 18440953.
- ↑ Vanek T, Jares M, Snircova J, Maly M (December 2007). "Fibrinolysis in coronary artery surgery: detection by thromboelastography". Interactive Cardiovascular and Thoracic Surgery. 6 (6): 700–4. doi: 10.1510/icvts.2007.161463 . PMID 17709365.
- ↑ Chapin JC, Hajjar KA (January 2015). "Fibrinolysis and the control of blood coagulation". Blood Reviews. 29 (1): 17–24. doi:10.1016/j.blre.2014.09.003. PMC 4314363 . PMID 25294122.
- ↑ Schöchl H (2008). "Hyperfibrinolysis:a prognostic marker of poor survival following major trauma?". Haemostaseologie. 28: A57.
- ↑ Marcos-Contreras, Oscar A.; Lizarrondo, Sara Martinez de; Bardou, Isabelle; Orset, Cyrille; Pruvost, Mathilde; Anfray, Antoine; Frigout, Yvann; Hommet, Yannick; Lebouvier, Laurent (2016-01-01). "Hyperfibrinolysis increases blood brain barrier permeability by a plasmin and bradykinin-dependent mechanism". Blood. 128 (20): blood–2016-03-705384. doi: 10.1182/blood-2016-03-705384 . ISSN 0006-4971. PMID 27531677.
- ↑ Vorweg M, Hartmann B, Knüttgen D, Jahn MC, Doehn M (December 2001). "Management of fulminant fibrinolysis during abdominal aortic surgery". Journal of Cardiothoracic and Vascular Anesthesia. 15 (6): 764–7. doi:10.1053/jcan.2001.28337. PMID 11748531.
- ↑ Diprose P, Herbertson MJ, O'Shaughnessy D, Deakin CD, Gill RS (March 2005). "Reducing allogeneic transfusion in cardiac surgery: a randomized double-blind placebo-controlled trial of antifibrinolytic therapies used in addition to intra-operative cell salvage". British Journal of Anaesthesia. 94 (3): 271–8. doi: 10.1093/bja/aei044 . PMID 15591329.