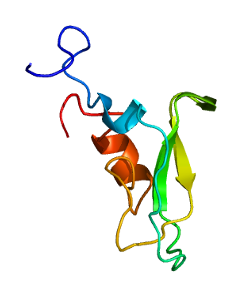
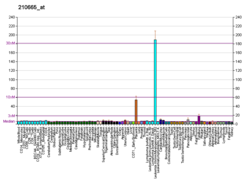
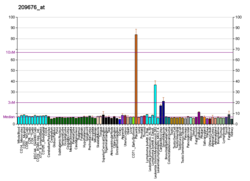
Tissue factor pathway inhibitor (or TFPI) is a single-chain polypeptide which can reversibly inhibit factor Xa (Xa). While Xa is inhibited, the Xa-TFPI complex can subsequently also inhibit the FVIIa-tissue factor complex. TFPI contributes significantly to the inhibition of Xa in vivo, despite being present at concentrations of only 2.5 nM.