Related Research Articles
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases. They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt its active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site.

Cathepsins are proteases found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover.
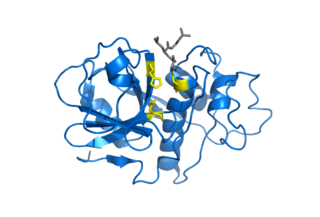
Cysteine proteases, also known as thiol proteases, are enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.

Cathepsin S is a protein that in humans is encoded by the CTSS gene. Transcript variants utilizing alternative polyadenylation signals exist for this gene.

Cathepsin O is an enzyme that in humans is encoded by the CTSO gene.

Cathepsin K, abbreviated CTSK, is an enzyme that in humans is encoded by the CTSK gene.
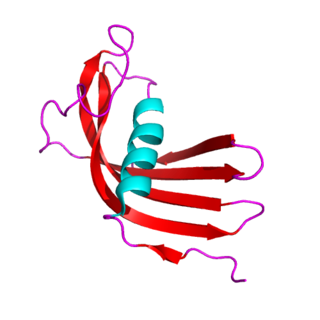
The cystatins are a family of cysteine protease inhibitors which share a sequence homology and a common tertiary structure of an alpha helix lying on top of an anti-parallel beta sheet. The family is subdivided as described below.

Cathepsin L1 is a protein that in humans is encoded by the CTSL1 gene.
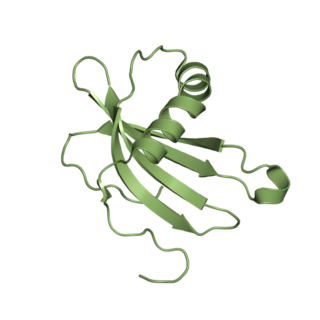
Cystatin-A is a protein that in humans is encoded by the CSTA gene.

Cystatin-B is a protein that in humans is encoded by the CSTB gene.
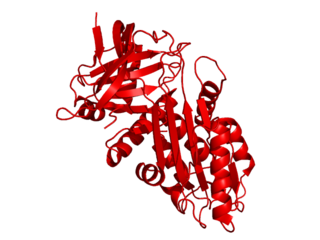
Serpin B3 is a protein that in humans is encoded by the SERPINB3 gene.

Leukocyte elastase inhibitor (LEI) also known as serpin B1 is a protein that in humans is encoded by the SERPINB1 gene. It is a member of the clade B serpins or ov-serpins founded by ovalbumin.

Cathepsin Z, also called cathepsin X or cathepsin P, is a protein that in humans is encoded by the CTSZ gene. It is a member of the cysteine cathepsin protease family, which has 11 members. As one of the 11 cathepsins, cathepsin Z contains distinctive features from others. Cathepsin Z has been reported involved in cancer malignancy and inflammation.

Cathepsin L2, also known as cathepsin V and encoded by the CTSV gene, is a human gene.

Cathepsin W is a protein that in humans is encoded by the CTSW gene.

Serpin B13 is a protein that in humans is encoded by the SERPINB13 gene.

Cathepsin F is a protein that in humans is encoded by the CTSF gene.
Angiogenesis is the process of forming new blood vessels from existing blood vessels. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteases (MMPs), a disintegrin and metalloprotease domain (ADAM), a disintegrin and metalloprotease domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
Uterine serpins are members of the A clade of the serine protease inhibitor (serpin) superfamily of proteins and are encoded by the SERPINA14 gene. Uterine serpins are produced by the endometrium of a restricted group of mammals under the influence of progesterone or estrogen. These proteins appear to be inactive protease inhibitors and may function during pregnancy to regulate immune function or participate in transplacental transport.
References
- ↑ McGowan S, Buckle AM, Irving JA, et al. (July 2006). "X-ray crystal structure of MENT: evidence for functional loop-sheet polymers in chromatin condensation". EMBO J. 25 (13): 3144–55. doi:10.1038/sj.emboj.7601201. PMC 1500978 . PMID 16810322.
- ↑ Irving JA, Shushanov SS, Pike RN, et al. (April 2002). "Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation". J. Biol. Chem. 277 (15): 13192–201. doi: 10.1074/jbc.M108460200 . PMID 11821386.