
Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.

The Urokinase receptor, also known as urokinase plasminogen activator surface receptor (uPAR) or CD87, is a protein encoded in humans by the PLAUR gene. It is a multidomain glycoprotein tethered to the cell membrane with a glycosylphosphotidylinositol (GPI) anchor. uPAR was originally identified as a saturable binding site for urokinase on the cell surface.

The immunoglobulin superfamily (IgSF) is a large protein superfamily of cell surface and soluble proteins that are involved in the recognition, binding, or adhesion processes of cells. Molecules are categorized as members of this superfamily based on shared structural features with immunoglobulins ; they all possess a domain known as an immunoglobulin domain or fold. Members of the IgSF include cell surface antigen receptors, co-receptors and co-stimulatory molecules of the immune system, molecules involved in antigen presentation to lymphocytes, cell adhesion molecules, certain cytokine receptors and intracellular muscle proteins. They are commonly associated with roles in the immune system. Otherwise, the sperm-specific protein IZUMO1, a member of the immunoglobulin superfamily, has also been identified as the only sperm membrane protein essential for sperm-egg fusion.

Activin A receptor, type I (ACVR1) is a protein which in humans is encoded by the ACVR1 gene; also known as ALK-2. ACVR1 has been linked to the 2q23-24 region of the genome. This protein is important in the bone morphogenic protein (BMP) pathway which is responsible for the development and repair of the skeletal system. While knock-out models with this gene are in progress, the ACVR1 gene has been connected to fibrodysplasia ossificans progressiva, a very rare progressive genetic disease characterized by heterotopic ossification of muscles, tendons and ligaments. It is a bone morphogenetic protein receptor, type 1.
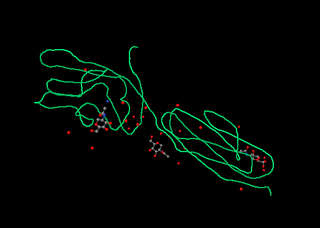
CD2 is a cell adhesion molecule found on the surface of T cells and natural killer (NK) cells. It has also been called T-cell surface antigen T11/Leu-5, LFA-2, LFA-3 receptor, erythrocyte receptor and rosette receptor.
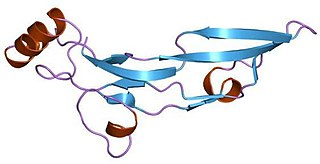
The transforming growth factor beta (TGF-β) superfamily is a large group of structurally related cell regulatory proteins that was named after its first member, TGF-β1, originally described in 1983. They interact with TGF-beta receptors.
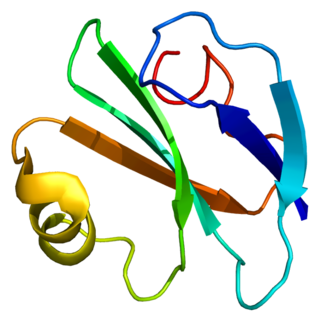
CD59 glycoprotein, also known as MAC-inhibitory protein (MAC-IP), membrane inhibitor of reactive lysis (MIRL), or protectin, is a protein that in humans is encoded by the CD59 gene. It is an LU domain and belongs to the LY6/uPAR/alpha-neurotoxin protein family.

Secreted Ly-6/uPAR-related protein 1 is a protein that in humans is encoded by the SLURP1 gene. It exerts anti-inflammatory effects, acts as a tumor suppressor, and antagonizes nicotinic receptors.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
Soluble urokinase plasminogen activator receptor (suPAR) is a protein and the soluble form of uPAR. uPAR is expressed mainly on immune cells, endothelial cells, and smooth muscle cells. uPAR is a membrane-bound receptor for uPA, also known as urokinase and Vitronectin. The soluble version of uPAR, called suPAR, results from the cleavage and membrane-bound uPAR during inflammation or immune activation. The suPAR concentration is positively correlated to the activation level of the immune system. Therefore, suPAR is a marker of disease severity and aggressiveness and is associated with morbidity and mortality in several acute and chronic diseases. suPAR levels have been observed to increase with age. suPAR is present in plasma, urine, blood, serum, and cerebrospinal fluid.
Somatomedin B is a serum factor of unknown function, is a small cysteine-rich peptide, derived proteolytically from the N-terminus of the cell-substrate adhesion protein vitronectin. Cys-rich somatomedin B-like domains are found in a number of proteins, including plasma-cell membrane glycoprotein and placental protein 11.
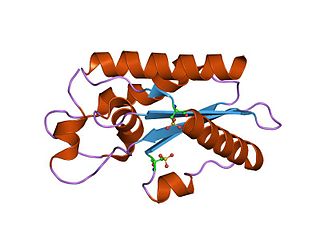
The CAP superfamily is a large superfamily of secreted proteins that are produced by a wide range of organisms, including prokaryotes and non-vertebrate eukaryotes.

Ly6/neurotoxin 1 is a protein in humans that is encoded by the LYNX1 gene. Alternatively spliced variants encoding different isoforms have been identified.
Lymphocyte antigen 6 complex, locus G6E (pseudogene) is a protein that in humans is encoded by the LY6G6E gene.
Ly6 also known as lymphocyte antigen 6 or urokinase-type plasminogen activator receptor (uPAR) is family of proteins that share a common structure but differ in their tissue expression patterns and function. Ly6 are cysteine-rich proteins that form disulfide bridges and contain a LU domain. These proteins are GPI-anchored to the cell membrane or are secreted. A total of 35 human and 61 mouse Ly6 family members have been identified. Depending on which tissues they are expressed in, LY6 family members have different roles. They are expressed in various types of tissues and their expression dependent on the stage of cell differentiation. For example, they are involved in cell proliferation, cell migration, cell–cell interactions, immune cell maturation, macrophage activation, and cytokine production. Their overexpression or dysregulation, for example due to point mutations, is associated with tumorogenesis and autoimmune diseases. This family was discovered in the 1970s, and these proteins are still used as markers of distinct stage of leukocyte differentiation.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).

Three-finger proteins or three-finger protein domains are a protein superfamily consisting of small, roughly 60-80 amino acid residue protein domains with a common tertiary structure: three beta strand loops extended from a hydrophobic core stabilized by disulfide bonds. The family is named for the outstretched "fingers" of the three loops. Members of the family have no enzymatic activity, but are capable of forming protein-protein interactions with high specificity and affinity. The founding members of the family, also the best characterized by structure, are the three-finger toxins found in snake venom, which have a variety of pharmacological effects, most typically by disruption of cholinergic signaling. The family is also represented in non-toxic proteins, which have a wide taxonomic distribution; 3FP domains occur in the extracellular domains of some cell-surface receptors as well as in GPI-anchored and secreted globular proteins, usually involved in signaling.

Irditoxin is a three-finger toxin (3FTx) protein found in the venom of the brown tree snake and likely in other members of the genus Boiga. It is a heterodimer composed of two distinct protein chains, each of the three-finger protein fold, linked by an intermolecular disulfide bond. This structure is unusual for 3FTx proteins, which are most commonly monomeric.

LY6/PLAUR Domain Containing 6B, also known under the name Cancer/Testis Antigen 116 (CTA116) and LYPD7 is encoded by the LYPD6B gene. LYPD6B is a member of the lymphocyte antigen 6 (LY6) protein family. It is expressed in the testis, lungs, stomach, prostate and in the nervous system where it acts as a modulator of nicotinic acetylcholine receptor (nAChRs) activity.













