
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Choline acetyltransferase is a transferase enzyme responsible for the synthesis of the neurotransmitter acetylcholine. ChAT catalyzes the transfer of an acetyl group from the coenzyme acetyl-CoA to choline, yielding acetylcholine (ACh). ChAT is found in high concentration in cholinergic neurons, both in the central nervous system (CNS) and peripheral nervous system (PNS). As with most nerve terminal proteins, ChAT is produced in the body of the neuron and is transported to the nerve terminal, where its concentration is highest. Presence of ChAT in a nerve cell classifies this cell as a "cholinergic" neuron. In humans, the choline acetyltransferase enzyme is encoded by the CHAT gene.

Donepezil, sold under the brand name Aricept among others, is a medication used to treat dementia of the Alzheimer's type. It appears to result in a small benefit in mental function and ability to function. Use, however, has not been shown to change the progression of the disease. Treatment should be stopped if no benefit is seen. It is taken by mouth or via a transdermal patch.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, Sjögren syndrome and underactive bladder.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.
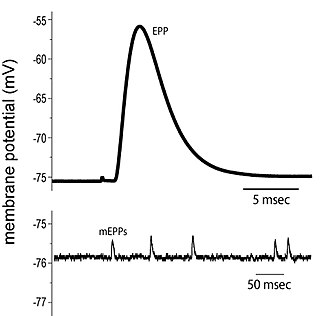
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
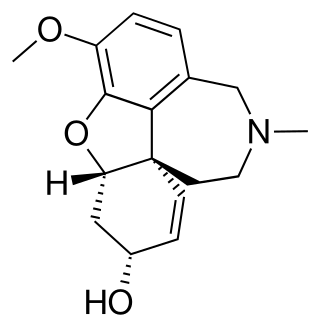
Galantamine is a type of acetylcholinesterase inhibitor. It is an alkaloid extracted from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

Methacholine, also known as acetyl-β-methylcholine, is a synthetic choline ester that acts as a non-selective muscarinic receptor agonist in the parasympathetic nervous system.

Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system (CNS) disorders.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

A cholinergic neuron is a nerve cell which mainly uses the neurotransmitter acetylcholine (ACh) to send its messages. Many neurological systems are cholinergic. Cholinergic neurons provide the primary source of acetylcholine to the cerebral cortex, and promote cortical activation during both wakefulness and rapid eye movement sleep. The cholinergic system of neurons has been a main focus of research in aging and neural degradation, specifically as it relates to Alzheimer's disease. The dysfunction and loss of basal forebrain cholinergic neurons and their cortical projections are among the earliest pathological events in Alzheimer's disease.
Autonomic drugs are substances that can either inhibit or enhance the functions of the parasympathetic and sympathetic nervous systems. This type of drug can be used to treat a wide range of diseases an disorders, including glaucoma, asthma, and disorders of the urinary, gastrointestinal and circulatory systems.

Phenserine is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.


















