
An opioidergic agent (or drug) is a chemical which directly or indirectly modulate the function of opioid receptors. Opioidergics comprise opioids, as well as allosteric modulators and enzyme affecting agents like enkephalinase inhibitors.

An opioidergic agent (or drug) is a chemical which directly or indirectly modulate the function of opioid receptors. Opioidergics comprise opioids, as well as allosteric modulators and enzyme affecting agents like enkephalinase inhibitors.


Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. Present in animals since early stages of evolution, in humans it plays roles in behavior that include social bonding, love, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during childbirth. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in maternal bonding and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.
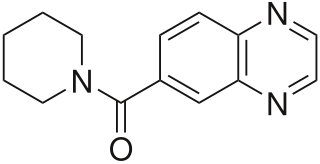
Ampakines or AMPAkines are a subgroup of AMPA receptor positive allosteric modulators with a benzamide or closely related chemical structure. They are also known as "CX compounds". Ampakines take their name from the AMPA receptor (AMPAR), a type of ionotropic glutamate receptor with which the ampakines interact and act as positive allosteric modulators (PAMs) of. Although all ampakines are AMPAR PAMs, not all AMPAR PAMs are ampakines.

Norbuprenorphine is a major active metabolite of the opioid modulator buprenorphine. It is a μ-opioid, δ-opioid, and nociceptin receptor full agonist, and a κ-opioid receptor partial agonist. In rats, unlike buprenorphine, norbuprenorphine produces marked respiratory depression but with very little antinociceptive effect. In explanation of these properties, norbuprenorphine has been found to be a high affinity P-glycoprotein substrate, and in accordance, shows very limited blood-brain-barrier penetration.

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the first letter of Morpheus, the compound's namesake in the original Greek. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.
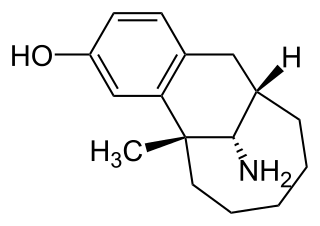
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.
DAMGO is a synthetic opioid peptide with high μ-opioid receptor specificity. It was synthesized as a biologically stable analog of δ-opioid receptor-preferring endogenous opioids, leu- and met-enkephalin. Structures of DAMGO bound to the μ opioid receptor reveal a very similar binding pose to morphinans.

Norketamine, or N-desmethylketamine, is the major active metabolite of ketamine, which is formed mainly by CYP3A4. Similarly to ketamine, norketamine acts as a noncompetitive NMDA receptor antagonist, but is about 3–5 times less potent as an anesthetic in comparison.

Hydroxynorketamine (HNK), or 6-hydroxynorketamine, is a minor metabolite of the anesthetic, dissociative, and antidepressant drug ketamine. It is formed by hydroxylation of the intermediate norketamine, another metabolite of ketamine. As of late 2019, (2R,6R)-HNK is in clinical trials for the treatment of depression.

Marta Filizola is a computational biophysicist who studies membrane proteins. Filizola's research concerns drug discovery the application of methods of computational chemistry and theoretical chemistry to biochemical and biomedical problems.
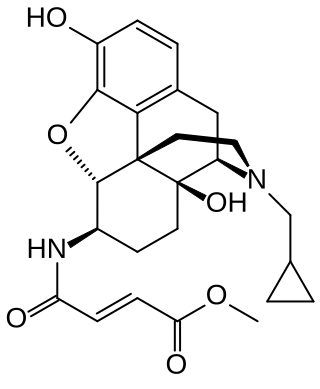
β-Funaltrexamine (β-FNA) is an irreversible opioid antagonist that was used to create the first crystal structure of the μ-opioid receptor (MOR). It is selective for antagonism of the MOR over the δ-opioid receptor (DOR) and κ-opioid receptor (KOR). Chemically, it is a naltrexone derivative with a methyl-fumaramide group in the 6-position. In addition to its MOR irreversible antagonism, β-FNA is a reversible agonist of the κ-opioid receptor (KOR) and produces KOR-mediated analgesic effects in animals. This has limited its usefulness and contributed to the development of methocinnamox as a more selective functionally irreversible antagonist of the MOR with no significant opioid agonistic actions.

Synthetic oxytocin, sold under the brand name Pitocin among others, is a medication made from the peptide oxytocin. As a medication, it is used to cause contraction of the uterus to start labor, increase the speed of labor, and to stop bleeding following delivery. For this purpose, it is given by injection either into a muscle or into a vein.

BMS‐986122 is a selective positive allosteric modulator (PAM) of the μ-opioid receptor (MOR).

Ignavine is a naturally occurring diterpene alkaloid found in Aconiti tuber. It has been reported to act as a μ-opioid receptor (MOR) positive allosteric modulator (PAM). The drug potentiated responses to the selective MOR agonist DAMGO at low concentrations but inhibited DAMGO at high concentrations. Ignavine alone has been found to produce analgesic effects in animals, but with a biphasic dose–response curve. Although described as a MOR PAM, other research suggests that ignavine is a ligand of the orthosteric site of the MOR and does not act as a PAM. Instead, it may be a MOR partial agonist. However, more research is necessary to clarify its MOR actions. Ignavine was first isolated by 1952 and its reported MOR PAM activity was first reported by 2016.

BMS-986187 is a positive allosteric modulator (PAM) of the δ-opioid receptor (DOR) and the κ-opioid receptor (KOR).

MS1 is a positive allosteric modulator (PAM) of the μ-opioid receptor (MOR). It was developed from structural modification of the earlier MOR PAM BMS‐986122.