Related Research Articles

Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common, life-threatening inherited human disorders and the most common hereditary kidney disease. It is associated with large interfamilial and intrafamilial variability, which can be explained to a large extent by its genetic heterogeneity and modifier genes. It is also the most common of the inherited cystic kidney diseases — a group of disorders with related but distinct pathogenesis, characterized by the development of renal cysts and various extrarenal manifestations, which in case of ADPKD include cysts in other organs, such as the liver, seminal vesicles, pancreas, and arachnoid membrane, as well as other abnormalities, such as intracranial aneurysms and dolichoectasias, aortic root dilatation and aneurysms, mitral valve prolapse, and abdominal wall hernias. Over 50% of patients with ADPKD eventually develop end stage kidney disease and require dialysis or kidney transplantation. ADPKD is estimated to affect at least one in every 1000 individuals worldwide, making this disease the most common inherited kidney disorder with a diagnosed prevalence of 1:2000 and incidence of 1:3000-1:8000 in a global scale.
Hyponatremia or hyponatraemia is a low concentration of sodium in the blood. It is generally defined as a sodium concentration of less than 135 mmol/L (135 mEq/L), with severe hyponatremia being below 120 mEq/L. Symptoms can be absent, mild or severe. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance. Severe symptoms include confusion, seizures, and coma; death can ensue.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.
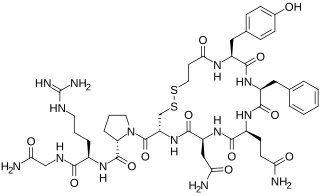
Desmopressin, sold under the trade name DDAVP among others, is a medication used to treat diabetes insipidus, bedwetting, hemophilia A, von Willebrand disease, and high blood urea levels. In hemophilia A and von Willebrand disease, it should only be used for mild to moderate cases. It may be given in the nose, by injection into a vein, by mouth, or under the tongue.
The syndrome of inappropriate antidiuretic hormone secretion (SIADH), also known as the syndrome of inappropriate antidiuresis (SIAD), is characterized by a physiologically inappropriate release of antidiuretic hormone (ADH) either from the posterior pituitary gland, or an abnormal non-pituitary source. Unsuppressed ADH causes a physiologically inappropriate increase in solute-free water being reabsorbed by the tubules of the kidney to the venous circulation leading to hypotonic hyponatremia.

Conivaptan, sold under the brand name Vaprisol, is a non-peptide inhibitor of the receptor for anti-diuretic hormone, also called vasopressin. It was approved in 2004 for hyponatremia. The compound was discovered by Astellas and marked in 2006. The drug is now marketed by Cumberland Pharmaceuticals, Inc.

Demeclocycline is a tetracycline antibiotic which was derived from a mutant strain of Streptomyces aureofaciens.
Primary polydipsia and psychogenic polydipsia are forms of polydipsia characterised by excessive fluid intake in the absence of physiological stimuli to drink. Psychogenic polydipsia which is caused by psychiatric disorders, often schizophrenia, is often accompanied by the sensation of dry mouth. Some forms of polydipsia are explicitly non-psychogenic. Primary polydipsia is a diagnosis of exclusion.

Vasopressin receptor 1A (V1AR), or arginine vasopressin receptor 1A is one of the three major receptor types for vasopressin, and is present throughout the brain, as well as in the periphery in the liver, kidney, and vasculature.

Vasopressin receptor 2 (V2R), or arginine vasopressin receptor 2, is a protein that acts as receptor for vasopressin. AVPR2 belongs to the subfamily of G-protein-coupled receptors. Its activity is mediated by the Gs type of G proteins, which stimulate adenylate cyclase.
The actions of vasopressin are mediated by stimulation of tissue-specific G protein-coupled receptors (GPCRs) called vasopressin receptors that are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. These three subtypes differ in localization, function and signal transduction mechanisms.

Tolvaptan, sold under the brand name Samsca among others, is an aquaretic drug that functions as a selective, competitive vasopressin receptor 2 (V2) antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan was approved by the U.S. Food and Drug Administration (FDA) on May 19, 2009, and is sold by Otsuka Pharmaceutical Co. under the trade name Samsca. Tolvaptan, as Jynarque, was granted approval for medical use in the United States in April 2018.
An aquaretic is a novel class of drug that is used to promote aquaresis, the excretion of water without electrolyte loss. Strictly speaking, aquaretics are not diuretics but are sometimes classified as such.

Satavaptan is a vasopressin-2 receptor antagonist which was investigation by Sanofi-Aventis and was under development for the treatment of hyponatremia. It was also being studied for the treatment of ascites. Development was discontinued in 2009.

Lixivaptan (VPA-985) is an orally-active, non-peptide, selective vasopressin 2 receptor antagonist being developed as an investigational drug by Palladio Biosciences, Inc. (Palladio), a subsidiary of Centessa Pharmaceuticals plc. As of December 2021, lixivaptan is in Phase III clinical development for the treatment of Autosomal dominant polycystic kidney disease (ADPKD), the most common form of polycystic kidney disease. The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to lixivaptan for the treatment of ADPKD.
Hypoosmolar hyponatremia is a condition where hyponatremia is associated with a low plasma osmolality. The term "hypotonic hyponatremia" is also sometimes used.

WAY-267464 is a potent, selective, non-peptide agonist for the oxytocin receptor, with negligible affinity for the vasopressin receptors. Contradictorily however, though originally described as selective for the oxytocin receptor and lacking affinity for the vasopressin receptors, it has since been reported to also act as a potent vasopressin V1A receptor antagonist. WAY-267464 has been shown to cross the blood–brain barrier to a significantly greater extent than exogenously applied oxytocin, and in animal tests produces centrally-mediated oxytocinergic actions such as anxiolytic effects, but with no antidepressant effect evident. It was developed by a team at Ferring Pharmaceuticals. WAY-267464 was under investigation for the potential clinical treatment of anxiety disorders by Wyeth, and reached the preclinical stage of development, but no development has been reported as of 2011.
A fluid restriction diet is a diet which limits the amount of daily fluid consumption. Besides beverages, many foods also include fluids which needs to be taken into consideration. A fluid-restrictive diet assists in preventing the build-up of fluids in the body. Reducing fluid intake can alleviate stress on the body and may reduce additional complications. A fluid restriction diet is generally medically advised for patients with "heart problems, renal disease, liver damage including cirrhosis, endocrine and adrenal gland issues, elevated stress hormones and hyponatremia". Patients with heart failure are recommended to restrict fluid intake down to 2 quarts per day.

Epelsiban is an orally bioavailable drug which acts as a selective and potent oxytocin receptor antagonist. It was initially developed by GlaxoSmithKline (GSK) for the treatment of premature ejaculation in men and then as an agent to enhance embryo or blastocyst implantation in women undergoing embryo or blastocyst transfer associated with in vitro fertilization (IVF)., and was also investigated for use in the treatment of adenomyosis.
TS-121 (THY1773) is an orally active, selective vasopressin V1B receptor antagonist which is under development by Taisho Pharmaceutical for the adjunctive treatment of major depressive disorder. As of May 2017, it is in phase II clinical trials for this indication.
References
- ↑ Palm C, Pistrosch F, Herbrig K, Gross P (July 2006). "Vasopressin antagonists as aquaretic agents for the treatment of hyponatremia". Am. J. Med. 119 (7 Suppl 1): S87–92. doi:10.1016/j.amjmed.2006.05.014. PMID 16843091.
- ↑ Serradeil-Le Gal C, Wagnon J, Valette G, Garcia G, Pascal M, Maffrand JP, Le Fur G (2002). "Chapter 15 Nonpeptide vasopressin receptor antagonists: Development of selective and orally active V1a, V2 and V1b receptor ligands". Vasopressin and Oxytocin: From Genes to Clinical Applications. Progress in Brain Research. Vol. 139. pp. 197–210. doi:10.1016/S0079-6123(02)39017-4. ISBN 978-0-444-50982-6. PMID 12436936.
- ↑ Lemmens-Gruber R, Kamyar M (2006). "Vasopressin antagonists". Cellular and Molecular Life Sciences. 63 (15): 1766–79. doi:10.1007/s00018-006-6054-2. PMID 16794787. S2CID 30709102.
- ↑ Decaux G, Soupart A, Vassart G (2008). "Non-peptide arginine-vasopressin antagonists: the vaptans". Lancet. 371 (9624): 1624–32. doi:10.1016/S0140-6736(08)60695-9. PMID 18468546. S2CID 1245079.
- ↑ Inhibition by somatostatin of the vasopressin-stimulated adenylate cyclase in a kidney-derived line of cells grown in defined medium. FEBS Letters. https://doi.org/10.1016/0014-5793(84)80304-X
- ↑ Padfield PL, Hodsman GP, Morton JJ (September 1978). "Demeclocycline in the treatment of the syndrome of inappropriate antidiuretic hormone release: with measurement of plasma ADH". Postgrad Med J. 54 (635): 623–7. doi:10.1136/pgmj.54.635.623. PMC 2425217 . PMID 103083.
- 1 2 Ajay K. Singh, Gordon H. Williams (12 January 2009). Textbook of Nephro-Endocrinology. Academic Press. pp. 250–251. ISBN 978-0-08-092046-7.
- 1 2 L. Kovács, B. Lichardus (6 December 2012). Vasopressin: Disturbed Secretion and Its Effects. Springer Science & Business Media. pp. 179–180. ISBN 978-94-009-0449-1.
- 1 2 3 4 5 6 7 8 9 10 Greenberg A, Verbalis JG (2006). "Vasopressin receptor antagonists". Kidney Int. 69 (12): 2124–30. doi: 10.1038/sj.ki.5000432 . PMID 16672911.
- 1 2 Ferguson JW, Therapondos G, Newby DE, Hayes PC (2003). "Therapeutic role of vasopressin receptor antagonism in patients with liver cirrhosis". Clin Sci (Lond). 105 (1): 1–8. doi:10.1042/CS20030062. PMID 12639215.