
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "green shoot" or "twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the international exhibition, which opened on 1 May that year.

Thallium(I) sulfate (Tl2SO4) or thallous sulfate is the sulfate salt of thallium in the common +1 oxidation state, as indicated by the Roman numeral I. It is often referred to as simply thallium sulfate.

Fluorometholone acetate, also known as oxylone acetate and sold under the brand names Flarex, Florate, and Omnitrol, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, as well as a progestogen and progestogen ester. It is the C17α acetate ester of fluorometholone.

Thallium(I) oxide is the inorganic compound of thallium and oxygen with the formula Tl2O in which thallium is in its +1 oxidation state. It is black and produces a basic yellow solution of thallium(I) hydroxide (TlOH) when dissolved in water. It is formed by heating solid TlOH or Tl2CO3 in the absence of air. Thallium oxide is used to make special high refractive index glass. Thallium oxide is a component of several high temperature superconductors. Thallium(I) oxide reacts with acids to make thallium(I) salts.

Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless salt is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.

Thallium(III) oxide, also known as thallic oxide, is a chemical compound of thallium and oxygen. It occurs in nature as the rare mineral avicennite. Its structure is related to that of Mn2O3 which has a bixbyite like structure. Tl2O3 is metallic with high conductivity and is a degenerate n-type semiconductor which may have potential use in solar cells. A method of producing Tl2O3 by MOCVD is known. Any practical use of thallium(III) oxide will always have to take account of thallium's poisonous nature. Contact with moisture and acids may form poisonous thallium compounds.

Thallium(I) sulfide, Tl2S, is a chemical compound of thallium and sulfur. It was used in some of the earliest photo-electric detectors by Theodore Case who developed the so-called thalofide (sometimes spelt thallofide) cell, used in early film projectors. Case described the detector material as consisting of thallium, oxygen and sulfur, and this was incorrectly described by others as being thallium oxysulfide, which incidentally is a compound that is not known. Case's work was then built on by R.J. Cashman who recognised that the controlled oxidation of the Tl2S film was key to the operation of the cell. Cashman's work culminated in the development of long wave infrared detectors used during the Second World War. Reliable Tl2S detectors were also developed in Germany at the same time.
Tl2S is found in nature as the mineral carlinite which has the distinction of being the only sulfide mineral of thallium that does not contain at least two metals. Tl2S has a distorted anti-CdI2 structure.
Tl2S can be prepared from the elements or by precipitating the sulfide from a solution of thallium(I), e.g. the sulfate or nitrate. Thin films have been deposited, produced from a mixture of citratothallium complex and thiourea. Heating the film in nitrogen at 300°C converts all the product into Tl2S

Metitepine, also known as methiothepin, is a drug described as a "psychotropic agent" of the tricyclic group which was never marketed. It acts as a non-selective antagonist of serotonin, dopamine, and adrenergic receptors and has antipsychotic properties.

Piperoxan, also known as benodaine, was the first antihistamine to be discovered. This compound, derived from benzodioxan, was prepared in the early 1930s by Daniel Bovet and Ernest Fourneau at the Pasteur Institute in France. Formerly investigated by Fourneau as an α-adrenergic-blocking agent, they demonstrated that it also antagonized histamine-induced bronchospasm in guinea pigs, and published their findings in 1933. Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution. One of Bovet and Fourneau's students, Anne-Marie Staub, published the first structure–activity relationship (SAR) study of antihistamines in 1939. Piperoxan and analogues themselves were not clinically useful due to the production of toxic effects in humans and were followed by phenbenzamine (Antergan) in the early 1940s, which was the first antihistamine to be marketed for medical use.
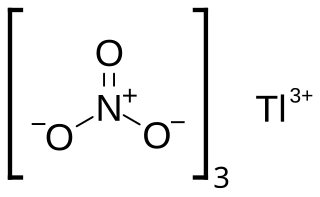
Thallium(III) nitrate, also known as thallic nitrate, is a thallium compound with chemical formula Tl(NO3)3. It is normally found as the trihydrate. It is a colorless and highly toxic salt. It is a strong oxidizing agent useful in organic synthesis. Among its many transformations, it oxidizes methoxyl phenols to quinone acetals, alkenes to acetals, and cyclic alkenes to ring-contracted aldehydes.
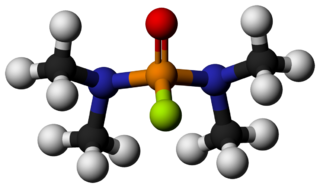
Dimefox, also known as TL-792 or T-2002, is a highly toxic organophosphate insecticide. In its pure form it is a colourless liquid with a fishy odour. Dimefox was first produced in 1940 by the group of Gerhard Schrader in Germany. It was historically used as a pesticide, but has been deemed obsolete or discontinued for use by the World Health Organization due to being an inhibitor of acetylcholinesterase. It is not guaranteed that all commercial use of this compound ceased, but in most countries it is no longer registered for use as a pesticide. It is considered an extremely hazardous substance as defined by the United States Emergency Planning and Community Right-to-Know Act.
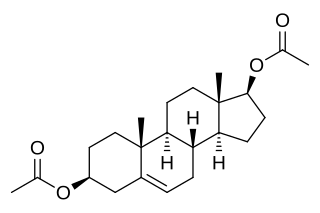
Androstenediol diacetate, or 5-androstenediol 3β,17β-diacetate, also known as androst-5-ene-3β,17β-diol 3β,17β-diacetate, is a synthetic anabolic-androgenic steroid and an androgen ester – specifically, the C3β,17β diacetate diester of 5-androstenediol (androst-5-ene-3β,17β-diol) – which was never marketed. The medication has been used with success to treat breast cancer in women.
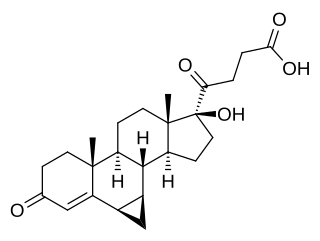
Prorenoic acid, or prorenoate, is a synthetic steroidal antimineralocorticoid which was never marketed.

T-1123 is a carbamate-based acetylcholinesterase inhibitor. It was investigated as a chemical warfare agent starting in 1940. It does not go through the blood-brain barrier due to the charge on quaternary nitrogen. The antidote is atropine. T-1123 is a quaternary ammonium ion. A phenyl carbamate ester is bonded in the meta position to the nitrogen on a diethylmethyl amine. The chloride and methylsulfate salt of T-1123 is TL-1299 and TL-1317, respectively.
Ethylsarin (GE), also known as EA-1209, TL-1620 or T-2109, is an organophosphate nerve agent of the G-series. It is the ethylphosphonofluoridate analog of sarin.

TL-1238 is an extremely potent carbamate acetylcholinesterase inhibitor. It has been shown to be more potent than neostigmine.

EA-3966 is a carbamate nerve agent. It is synthesized by reacting 2-dimethylaminomethyl-3-dimethylcarbamoxypyridine with 10-bromodecyltrimethylammonium bromide.

TL-599, also known as SB-8, is an extremely potent carbamate acetylcholinesterase inhibitor.

T-1152 is a quaternary carbamate anticholinesterase. It is synthesized by reaction of m-dimethylaminophenol with methyl isocyanate, followed by quaternization with methyl iodide. Since T-1152 is toxic by ingestion, it was patented as a rodenticide in 1932.



















