
Mustard gas or sulfur mustard is any of several chemical compounds that contain the chemical structure S(CH2CH2Cl)2. In the wider sense, compounds with the substituent S(CH2CH2X)2 and N(CH2CH2X)3 are known as sulfur mustards and nitrogen mustards, respectively, where X = Cl or Br. Such compounds are potent alkylating agents, which can interfere with several biological processes. Also known as mustard agents, this family of compounds are infamous cytotoxins and blister agents with a long history of use as chemical weapons. The name mustard gas is technically incorrect: the substances, when dispersed, are often not gases but a fine mist of liquid droplets. Sulfur mustards are viscous liquids at room temperature and have an odor resembling mustard plants, garlic, or horseradish, hence the name. When pure, they are colorless, but when used in impure forms, such as in warfare, they are usually yellow-brown. Mustard gases form blisters on exposed skin and in the lungs, often resulting in prolonged illness ending in death. The typical mustard gas is the organosulfur compound bis(2-chloroethyl) sulfide.

Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a chemical weapon, acting as a vesicant and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to geraniums.

A blister agent, is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as cantharidin are also blister-producing agents (vesicants). Furanocoumarin, another naturally occurring substance, causes vesicant-like effects indirectly, for example, by increasing skin photosensitivity greatly. Vesicants have medical uses including wart removal but can be dangerous if even small amounts are ingested.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Chlormethine, also known as mechlorethamine, mustine, HN2, and embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent, which is a type of blister agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable and irritating odor. It is used as a reagent in organic chemistry.

Nitrogen mustards (NMs) are cytotoxic organic compounds with the bis(2-chloroethyl)amino ((ClC2H4)2NR) functional group. Although originally produced as chemical warfare agents, they were the first chemotherapeutic agents for treatment of cancer. Nitrogen mustards are nonspecific DNA alkylating agents.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).
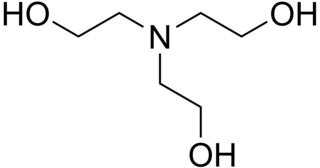
Triethanolamine, or TEOA, is an organic compound with the chemical formula N(CH2CH2OH)3. It is a colourless, viscous liquid. It is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples may appear yellow because of impurities.
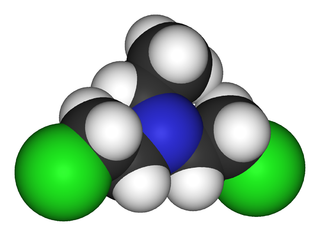
Bis(2-chloroethyl)ethylamine is the organic compound with the formula C2H5N(CH2CH2Cl)2. Often abbreviated HN1, it is a powerful vesicant and a nitrogen mustard gas used for chemical warfare. HN1 was developed in the 1920s and 1930s to remove warts and later as a military agent. Because of the latter use, it is a Schedule 1 chemical within the Chemical Weapons Convention and therefore use and production is strongly restricted. It has never been used in warfare.

Sesquimustard is the organosulfur compound with the formula (ClCH2CH2SCH2)2. Although it is a colorless solid, impure samples are often brown. The compound is a type of mustard gas, a vesicant used as a chemical weapon. From the chemical perspective, the compound is both a thioether and an alkyl chloride.
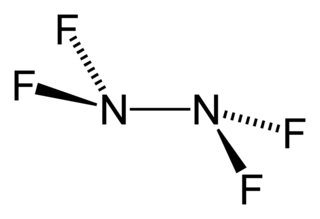
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable, reactive inorganic gas. It is a fluorinated analog of hydrazine.

Tris(2-aminoethyl)amine is the organic compound with the formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Tris(2-aminoethyl)amine is commonly abbreviated as tren or TREN. It is used a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry.

Phenyldichloroarsine, also known by its wartime name phenyl Dick and its NATO abbreviation PD, is an organic arsenical vesicant and vomiting agent developed by Germany and France for use as a chemical warfare agent during World War I. The agent is known by multiple synonyms and is technically classified as a vesicant, or blister agent.
O-Mustard (T) is a vesicant chemical weapon, a type of mustard gas, with around 3 times the toxicity of the original sulfur mustard. It was developed in England in the 1930s as a thickener for mustard gas to make it more persistent when used in warm climates. A mixture of 60% sulfur mustard and 40% O-mustard also has a lower freezing point than pure sulfur mustard, and was given the code name HT. O-mustard is a Schedule I substance under the Chemical Weapons Convention.

Bis(2-chloroethyl)sulfide is the organosulfur compound with the formula (ClCH2CH2)2S. It is a prominent member of a family of cytotoxic and blister agents known as mustard agents. Sometimes referred to as mustard gas, the term is technically incorrect: bis(2-chloroethyl)sulfide is a liquid at room temperature. In warfare it was dispersed in the form of a fine mist of liquid droplets.
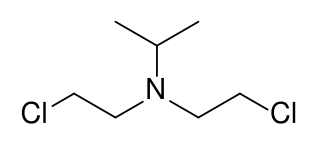
TL-301 is a nitrogen mustard vesicant.
















