
A cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Hydrogen cyanide (HCN), sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.

Lactic acid is an organic acid. It has a molecular formula CH3CH(OH)COOH. It is white in solid state and it is miscible with water. While in liquid state (dissolved state) it is a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate.

Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building-block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
Cyanogen iodide or iodine cyanide (ICN) is a pseudohalogen composed of iodine and the cyanide group. It is a relatively volatile and highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form hydrogen cyanide.

Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, an important industrial polymer.

Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is also used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Adiponitrile is the organic compound with the formula (CH2)4(CN)2. This dinitrile, a viscous, colourless liquid, is an important precursor to the polymer nylon-6,6. In 2005, about one million tonnes were produced.
2-Chloroethanol is a chemical compound with the formula HOCH2CH2Cl and the simplest chlorohydrin. This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional groups.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
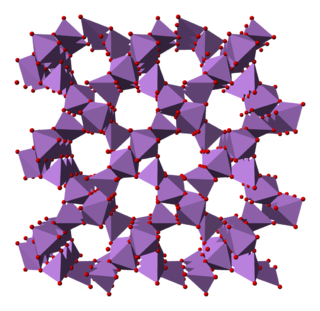
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.

Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Chloromethyl methyl ether (CMME) is a compound with formula CH3OCH2Cl. It is a chloroalkyl ether. It is used as an alkylating agent and industrial solvent to manufacture the detergent dodecylbenzyl chloride, water repellents and ion-exchange resins. In organic synthesis, it is used for introducing the methoxymethyl (MOM) protecting group, and is thus often called MOM-Cl or MOM chloride. It also finds application as a chloromethylating agent in some variants of the Blanc chloromethylation.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space.

Bis(chloromethyl) ketone is a chemical substance with formula C
3H
4Cl
2O. It is a solid, and is used in the making of citric acid. Exposures such as contact or inhalation of bis(chloromethyl) ketone can result in irritation or damage to skin, eyes, throat, lungs, liver and kidneys, as well as headaches and fainting.

3,4-Dichlorophenyl isocyanate is a chemical compound used as a chemical intermediate and in organic synthesis. It is a solid, and ranges in colour from white to yellow. It is an irritant for tissues including eyes and mucous membranes, and inhalation of dust from the chemical is poisonous. It can be used industrially in the preparation of triclocarban by reaction with p-chloroaniline.












