
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Chloroacetophenone is thermally stable, and is the only tear agent that is distillable at ambient conditions.
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917 in Robinson's Tropinone total synthesis. Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963. E.J. Corey formalized and popularized the concept from 1967 onwards in his article General methods for the construction of complex molecules and his book The Logic of Chemical Synthesis.

Carbon tetrabromide, CBr4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature.
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odor.
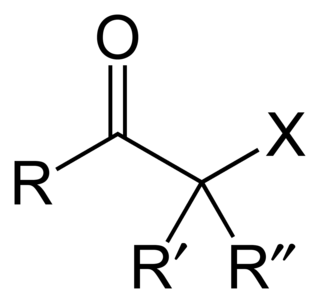
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.

Copper(I) bromide is the chemical compound with the formula CuBr. This white diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.

Phenylglyoxal is the organic compound with the formula C6H5C(O)C(O)H. It contains both an aldehyde and a ketone functional group. It is yellow liquid when anhydrous but readily forms a colorless crystalline hydrate. It has been used as a reagent to modify the amino acid, arginine. It has also been used to attach chemical payload (probes) to the amino acid citrulline and to peptides/proteins.

1,2-Dibromopropane, also known as propylene dibromide, is an organic compound with the formula CH3CHBrCH2Br. It is the simplest chiral hydrocarbon containing two bromine atoms:

Bromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808.

Vinyl bromide is the organobromine compound with the formula CH2=CHBr. Classified as a vinyl halide, it is a colorless gas at room temperature. It is used as a reagent and a comonomer.
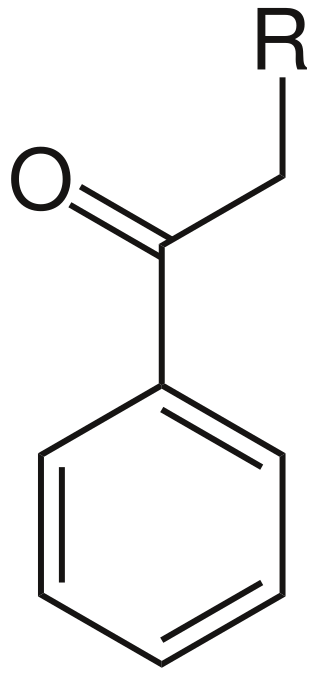
In organic chemistry, a phenacyl group is an aromatic substituent that consists of a phenyl group attached to an acyl group. A molecule containing a phenacyl group has the formula RCH2(CO)C6H5 and the structure shown to the right. Here, R denotes the remainder of the molecule; for instance, if R is Br, then the compound could be called "phenacyl bromide". Note however that in the standard IUPAC nomenclature this compound would instead be called "2-bromo-1-phenylethanone".
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

Benzanilide is the organic compound with the formula C6H5C(O)NHC6H5. It is a white solid. Commercially available, it may be prepared by treating benzoic acid with aniline.

Dihydrochalcone (DHC) is the organic compound with the formula C6H5C(O)(CH2)2C6H5. It is the reduced derivative of chalcone (C6H5C(O)(CH)2C6H5). It is a white solid that is soluble in many organic solvents. Dihydrochalcone per se is often minor significance, but some derivatives occur in nature and have attracted attention as drugs.

Thiobenzoic acid is an organosulfur compound with molecular formula C6H5COSH. It is the parent of aryl thiocarboxylic acids. It is a pale yellow liquid that freezes just below room temperature.

Xylylene dibromide is an organic compound with the formula C6H4(CH2Br)2. It is an off-white solid that, like other benzyl halides, is strongly lachrymatory. It is a useful reagent owing to the convenient reactivity of the two C-Br bonds. Two other isomers are known, para- and meta-xylylene dibromide.


















