 | |
| Names | |
|---|---|
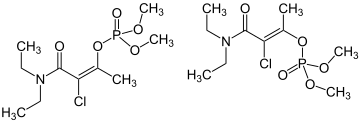
| IUPAC name (E/Z)-[3-Chloro-4-(diethylamino)-4-oxobut-2-en-2-yl] dimethyl phosphate | |
| Other names Dimecron | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.818 |
| KEGG | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H19ClNO5P | |
| Molar mass | 299.69 g·mol−1 |
| Density | 1.2132 g/cm3 [1] |
| Boiling point | 162 °C (324 °F; 435 K) (1.5 mmHg) [2] |
| Miscible | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 13 mg/kg (mouse, oral) [3] 6 mg/kg (mouse, IV) [3] 20 mg/kg (rat, oral) [3] 26 mg/kg (rat, subcut.) [3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Phosphamidon is an organophosphate insecticide first reported in 1960. [3] [2] It acts as a cholinesterase inhibitor.
The commercial product typically exists as a mixture of 70% (Z)-isomer and 30% (E)-isomer. [1]