 | |
 Chlorantraniliprole 3D molecular model generated using Avogadro software | |
| Names | |
|---|---|
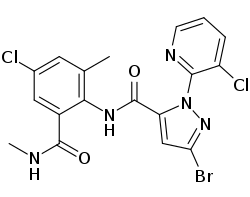
| Preferred IUPAC name 3-Bromo-N-[4-chloro-2-methyl-6-(methylcarbamoyl)phenyl]-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamide | |
| Other names Rynaxypyr, Coragen, Altacor | |
| Identifiers | |
3D model (JSmol) | |
| 11247880 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.112.607 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C18H14BrCl2N5O2 | |
| Molar mass | 483.15 g·mol−1 |
| Melting point | 209 °C (408 °F; 482 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H319, H335, H410 | |
| P261, P264, P271, P273, P280, P304+P340, P305+P351+P338, P312, P337+P313, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Chlorantraniliprole is an insecticide of the diamide class used for insects found on fruit and vegetable crops as well as ornamental plants. [1]
Chlorantraniliprole opens muscular calcium channels, in particular the ryanodine receptor, rapidly causing paralysis and ultimately death of sensitive species (IRAC class 28). The differential selectivity chlorantraniliprole has towards insect ryanodine receptors explains its low mammalian toxicity receptor. [2] Chlorantraniliprole is active on chewing pest insects primarily by ingestion and secondarily by contact.