
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Acaricides, which kill mites and ticks, are not strictly insecticides, but are usually classified together with insecticides. The major use of Insecticides is agriculture, but they are also used in home and garden, industrial buildings, vector control and control of insect parasites of animals and humans. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain.

Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. It is a non-systemic and non-volatile insecticide that acts by contact and ingestion, used in agriculture and in pest control products. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, ant chalk, and some products of Baygon in Southeast Asia.
Pyrethrum was a genus of several Old World plants now classified in either Chrysanthemum or Tanacetum which are cultivated as ornamentals for their showy flower heads. Pyrethrum continues to be used as a common name for plants formerly included in the genus Pyrethrum. Pyrethrum is also the name of a natural insecticide made from the dried flower heads of Chrysanthemum cinerariifolium and Chrysanthemum coccineum. The insecticidal compounds present in these species are pyrethrins.

Piperonyl butoxide (PBO) is a pale yellow to light brown liquid organic compound used as an adjuvant component of pesticide formulations for synergy. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. It is a semisynthetic derivative of safrole and is produced from the condensation of the sodium salt of 2-(2-butoxyethoxy) ethanol and the chloromethyl derivative of hydrogenated safrole (dihydrosafrole); or through 1,2-Methylenedioxybenzene.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.

Bifenthrin is a pyrethroid insecticide. It is widely used against ant infestations.
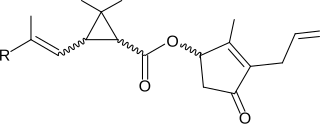
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums. Pyrethroids are used as commercial and household insecticides.

The allethrins are a group of related synthetic compounds used in insecticides. They are classified as pyrethroids, i.e. synthetic versions of pyrethrin, a chemical with insecticidal properties found naturally in Chrysanthemum flowers. They were first synthesized in the United States by Milton S. Schechter in 1949. Allethrin was the first pyrethroid.

Deltamethrin is a pyrethroid ester insecticide. Deltamethrin plays a key role in controlling malaria vectors, and is used in the manufacture of long-lasting insecticidal mosquito nets; however, resistance of mosquitos and bed bugs to deltamethrin has seen a widespread increase.

Resmethrin is a pyrethroid insecticide with many uses, including control of the adult mosquito population.
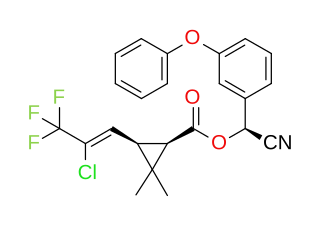
Cyhalothrin is an organic compound that, in specific isomeric forms, is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as cyhalothrin are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.

Indoxacarb is an oxadiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. It is also used as the active ingredient in the Syngenta line of commercial pesticides: Advion and Arilon.

Methomyl is a carbamate insecticide introduced in 1966. It is highly toxic to humans, livestock, pets, and wildlife. The EU imposed a pesticide residue limit of 0,01 mg/kg for all fruit and vegetables.
A fogger is any device that creates a fog, typically containing an insecticide for killing insects and other arthropods. Foggers are often used by consumers as a low cost alternative to professional pest control services. The number of foggers needed for pest control depends on the size of the space to be treated, as stated for safety reasons on the instructions supplied with the devices. The fog may contain flammable gases, leading to a danger of explosion if a fogger is used in a building with a pilot light or other naked flame.

Phoxim is an organophosphate insecticide that is produced by the Bayer corporation. It is an analogous dimethyl ester and an organothiophosphate acaricide. It is allowed for use in limited applications in the European Union. It is banned for use on crops in the European Union since 22 December 2007.

Esfenvalerate is a synthetic pyrethroid insecticide marketed under the brand Asana. It is the (S)-enantiomer of fenvalerate.

Cyfluthrin is a pyrethroid insecticide and common household pesticide. It is a complex organic compound and the commercial product is sold as a mixture of isomers. Like most pyrethroids, it is highly toxic to fish and invertebrates, but it is far less toxic to humans. It is generally supplied as a 10–25% liquid concentrate for commercial use and is diluted prior to spraying onto agricultural crops and outbuildings.

Helicoverpa assulta, the oriental tobacco budworm, is a moth of the family Noctuidae. H. assulta adults are migratory and are found all over the Old World Tropics including Asia, Africa, and Australia.

Tefluthrin is the ISO common name for an organic compound that is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as tefluthrin are often preferred as active ingredients in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. It is effective against soil pests because it can move as a vapour without irreversibly binding to soil particles: in this respect it differs from most other pyrethroids.

Fenpropathrin, or fenopropathrin, is a widely used pyrethroid insecticide in agriculture and household. Fenpropathrin is an ingestion and contact synthetic pyrethroid. Its mode of action is similar to other natural (pyrethrum) and synthetic pyrethroids where in they interfere with the kinetics of voltage gated sodium channels causing paralysis and death of the pest. Fenpropathrin was the first of the light-stable synthetic pyrethroids to be synthesized in 1971, but it was not commercialized until 1980. Like other pyrethroids with an α-cyano group, fenpropathrin also belongs to the termed type II pyrethroids. Type II pyrethroids are a more potent toxicant than type I in depolarizing insect nerves. Application rates of fenpropathrin in agriculture according to US environmental protection agency (EPA) varies by crop but is not to exceed 0.4 lb ai/acre.

















