
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-born diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.

Triclopyr is an organic compound in the pyridine group that is used as a systemic foliar herbicide and fungicide.
A reference dose is the United States Environmental Protection Agency's maximum acceptable oral dose of a toxic substance.Reference doses are most commonly determined for pesticides. The EPA defines an oral reference dose as:
[A]n estimate, with uncertainty spanning perhaps an order of magnitude, of a daily oral exposure to the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime.

Chlorpyrifos (CPS), also known as Chlorpyrifos ethyl, is an organophosphate pesticide used on crops, animals, and buildings, and in other settings, to kill a number of pests, including insects and worms. It acts on the nervous systems of insects by inhibiting the acetylcholinesterase enzyme. Chlorpyrifos was patented in 1966 by Dow Chemical Company.
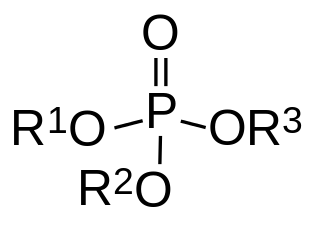
Organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to different polymers made OPEs to be widely used in different industries including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physically rather than by chemical bond. Due to this, OPEs leak into the environment more readily through volatilization, leaching, and abrasion. OPEs have been detected in different environmental compartments such as air, dust, water, sediment, soil and biota samples at higher frequency and concentration.
Pesticides vary in their effects on bees. Contact pesticides are usually sprayed on plants and can kill bees when they crawl over sprayed surfaces of plants or other areas around it. Systemic pesticides, on the other hand, are usually incorporated into the soil or onto seeds and move up into the stem, leaves, nectar, and pollen of plants.

Diazinon, a colorless to dark brown liquid, is a thiophosphoric acid ester developed in 1952 by Ciba-Geigy, a Swiss chemical company. It is a nonsystemic organophosphate insecticide formerly used to control cockroaches, silverfish, ants, and fleas in residential, non-food buildings. Diazinon was heavily used during the 1970s and early 1980s for general-purpose gardening use and indoor pest control. A bait form was used to control scavenger wasps in the western U.S. Diazinon is used in flea collars for domestic pets in Australia and New Zealand. Residential uses of diazinon were outlawed in the U.S. in 2004 because of human health risks but it is still approved for agricultural uses. An emergency antidote is atropine.
The allethrins are a group of related synthetic compounds used in insecticides. They are classified as pyrethroids, i.e. synthetic versions of pyrethrin, a chemical with insecticidal properties found naturally in Chrysanthemum flowers. They were first synthesized in the United States by Milton S. Schechter in 1949. Allethrin was the first pyrethroid.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.
Phenothrin, also called sumithrin and d-phenothrin, is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humans. d-Phenothrin is used as a component of aerosol insecticides for domestic use. It is often used with methoprene, an insect growth regulator that interrupts the insect's biological lifecycle by killing the eggs.

Baygon is a pesticide brand produced by S. C. Johnson & Son. It is an insecticide used for extermination and control of household pests such as crickets, roaches, ants, carpenter ants, spiders, silverfish and mosquitoes. In 1975, Baygon introduced Australia’s first surface spray for killing cockroaches, ticks and other crawling insects.

Organophosphate poisoning is poisoning due to organophosphates (OPs). Organophosphates are used as insecticides, medications, and nerve agents. Symptoms include increased saliva and tear production, diarrhea, vomiting, small pupils, sweating, muscle tremors, and confusion. While onset of symptoms is often within minutes to hours, some symptoms can take weeks to appear. Symptoms can last for days to weeks.

The Food Quality Protection Act (FQPA), or H.R.1627, was passed unanimously by Congress in 1996 and was signed into law by President Bill Clinton on August 3, 1996. The FQPA standardized the way the Environmental Protection Agency (EPA) would manage the use of pesticides and amended the Federal Insecticide, Fungicide, and Rodenticide Act and the Federal Food Drug and Cosmetic Act. It mandated a health-based standard for pesticides used in foods, provided special protections for babies and infants, streamlined the approval of safe pesticides, established incentives for the creation of safer pesticides, and required that pesticide registrations remain current.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
This is an index of articles relating to pesticides.

Health effects of pesticides may be acute or delayed in those who are exposed. A 2007 systematic review found that "most studies on non-Hodgkin lymphoma and leukemia showed positive associations with pesticide exposure" and thus concluded that cosmetic use of pesticides should be decreased. Strong evidence also exists for other negative outcomes from pesticide exposure including neurological problems, birth defects, fetal death, and neurodevelopmental disorder.

Bed bugs, or Cimicidae, are small parasitic insects. The term usually refers to species that prefer to feed on human blood.
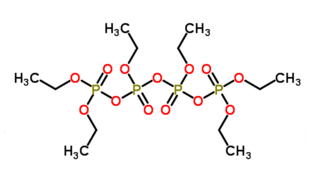
Hexaethyl tetraphosphate (also known as HET) is the organophosphorus compound with the chemical formula [(C2H5O)3P2O3]2O. The compound has not been isolated in pure form but appears to be a colorless liquid at room temperature. Commercial samples appear brown due to impurities. It is a constituent of the insecticide Bladan. In the 1940s, it was about as significant an insecticide as DDT and was referred to as "another of DDT's rivals for fame" in a 1948 book.















