
An acetylcholine receptor or a cholinergic receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.
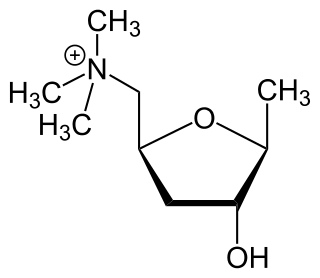
Muscarinic acetylcholine receptors (mAChRs) are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers. They are mainly found in the parasympathetic nervous system, but also have a role in the sympathetic nervous system in the control of sweat glands.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine occurs naturally remains controversial due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Tubocurarine is a toxic benzylisoquinoline alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. Safer alternatives, such as cisatracurium and rocuronium, have largely replaced it as an adjunct for clinical anesthesia and it is now rarely used.
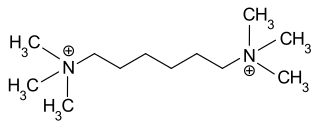
Hexamethonium is a non-depolarising ganglionic blocker, a neuronal nicotinic (nAChR) receptor antagonist that acts in autonomic ganglia by binding mostly in or on the nAChR receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system, nor on the nicotinic receptors at the skeletal neuromuscular junction, but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (nAChR).
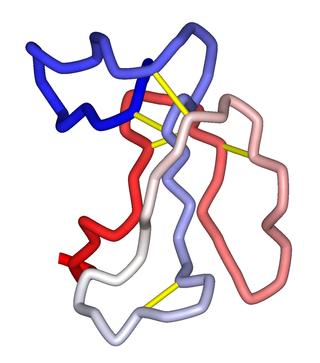
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Methyllycaconitine was identified one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralysis, and it has been shown to have insecticidal properties. It has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.
A ganglionic blocker is a type of medication that inhibits transmission between preganglionic and postganglionic neurons in the autonomic nervous system, often by acting as a nicotinic receptor antagonist. Nicotinic acetylcholine receptors are found on skeletal muscle, but also within the route of transmission for the parasympathetic and sympathetic nervous system. More specifically, nicotinic receptors are found within the ganglia of the autonomic nervous system, allowing outgoing signals to be transmitted from the presynaptic to the postsynaptic cells. Thus, for example, blocking nicotinic acetylcholine receptors blocks both sympathetic (excitatory) and parasympathetic (calming) stimulation of the heart. The nicotinic antagonist hexamethonium, for example, does this by blocking the transmission of outgoing signals across the autonomic ganglia at the postsynaptic nicotinic acetylcholine receptor.

α-Cobratoxin is a substance of the venom of certain Naja cobras. It is a nicotinic acetylcholine receptor (nAChR) antagonist which causes paralysis by preventing the binding of acetylcholine to the nAChR.
The muscle-type nicotinic receptor is a type of nicotinic acetylcholine receptor consisting of the subunit combination (α1)2β1δε (adult receptor) or (α1)2β1δγ (fetal receptor). These receptors are found in neuromuscular junctions, where activation leads to an excitatory postsynaptic potential (EPSP), mainly by increased Na+ and K+ permeability.
Pentolinium is a ganglionic blocking agent which acts as a nicotinic acetylcholine receptor antagonist. Formulated as the pentolinium tartrate salt, it is also known as Ansolysen. It can be used as an antihypertensive drug during surgery or to control hypertensive crises. It works by binding to the acetylcholine receptor of adrenergic nerves and thereby inhibiting the release of noradrenaline and adrenaline. Blocking this receptor leads to smooth muscle relaxation and vasodilation.
Philanthotoxins are components of the venom of the Egyptian solitary wasp Philanthus triangulum, commonly known as the European beewolf. Philanthotoxins are polyamine toxins, a group of toxins isolated from the venom of wasps and spiders which immediately but reversibly paralyze their prey. δ-philanthotoxin, also known as PhTX-433, is the most active philanthotoxin that can be refined from the venom. PhTX-433 functions by non-selectively blocking excitatory neurotransmitter ion channels, including nicotinic acetylcholine receptors (nAChRs) and ionotropic glutamate receptors (iGluRs). Synthetic analogues, including PhTX-343 and PhTX-12, have been developed to improve selectivity. While the IC50 values of philanthotoxins varies between analogues and receptor subunit composition, the IC50 value of PhTX-433 at the iGluR AMPA receptor naturally expressed in locust leg muscle is 18 μM and the IC50 value at rat nAChRs is 1 μM.
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.

Phantasmidine is a toxic substance derived from the Ecuadorian poisonous frog Anthony's poison arrow frog, more commonly known as the “phantasmal poison frog”. It is a nicotinic agonist, meaning it binds to nicotinic receptors in the body and mimics the effects of the neurotransmitter acetylcholine. This causes the stimulation of the body's parasympathetic nervous system, which induces many inhibitory behaviors in the body such as decreased heart rate.
The alpha-3 beta-2 nicotinic receptor, also known as the α3β2 receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β2 subunits.

κ-Bungarotoxin is a protein neurotoxin of the bungarotoxin family that is found in the venom of the many-banded krait, a snake found in Taiwan. κ-Bungarotoxin is a high affinity antagonist of nicotinic acetylcholine receptors (nAChRs), particularly of CHRNA3; it causes a post-synaptic blockade of neurotransmission. Although there is significant variability in the clinical effects of snake bites, neuromuscular paralysis and respiratory failure are associated with krait bites.

Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.

Babylonia japonica, common name the Japanese Babylon or Japanese ivory shell, is a species of sea snail, a marine gastropod mollusc in the family Babyloniidae.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.














