Related Research Articles

Phagocytosis is the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte.

Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system. They belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cell and other intracellular pathogens acting at around 3 days after infection, and respond to tumor formation. Most immune cells detect the antigen presented on major histocompatibility complex (MHC) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
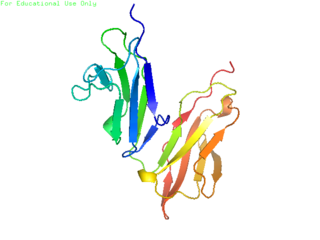
CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
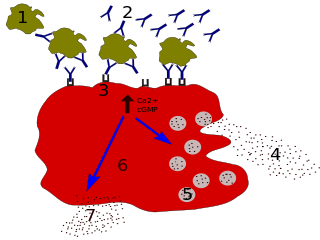
Degranulation is a cellular process that releases antimicrobial cytotoxic or other molecules from secretory vesicles called granules found inside some cells. It is used by several different cells involved in the immune system, including granulocytes. It is also used by certain lymphocytes such as natural killer (NK) cells and cytotoxic T cells, whose main purpose is to destroy invading microorganisms.
CD64 is a type of integral membrane glycoprotein known as an Fc receptor that binds monomeric IgG-type antibodies with high affinity. It is more commonly known as Fc-gamma receptor 1 (FcγRI). After binding IgG, CD64 interacts with an accessory chain known as the common γ chain, which possesses an ITAM motif that is necessary for triggering cellular activation.

Low affinity immunoglobulin gamma Fc region receptor III-A is a protein that in humans is encoded by the FCGR3A gene. It is also known as CD16a as it is part of the cluster of differentiation cell surface molecules.
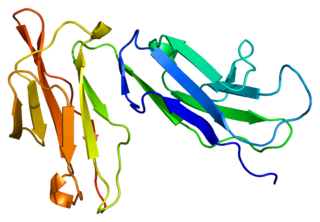
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.

Fc fragment of IgA receptor (FCAR) is a human gene that codes for the transmembrane receptor FcαRI, also known as CD89. FcαRI binds the heavy-chain constant region of Immunoglobulin A (IgA) antibodies. FcαRI is present on the cell surface of myeloid lineage cells, including neutrophils, monocytes, macrophages, and eosinophils, though it is notably absent from intestinal macrophages and does not appear on mast cells. FcαRI plays a role in both pro- and anti-inflammatory responses depending on the state of IgA bound. Inside-out signaling primes FcαRI in order for it to bind its ligand, while outside-in signaling caused by ligand binding depends on FcαRI association with the Fc receptor gamma chain.

FCGR3B, also known as CD16b, is a human gene.
The following outline is provided as an overview of and topical guide to immunology:
Urelumab is a fully human, non‐ligand binding, CD137 agonist immunoglobulin‐γ 4 (IgG4) monoclonal antibody. It was developed utilizing Medarex's UltiMAb(R) technology by Bristol-Myers Squibb for the treatment of cancer and solid tumors. Urelumab promotes anti-tumor immunity, or an immune response against tumor cells, via CD137 activation. The application of Urelumab has been limited due to the fact that it can cause severe liver toxicity.

Killer Activation Receptors (KARs) are receptors expressed on the plasmatic membrane of Natural Killer cells. KARs work together with inhibitory receptors, which inactivate them in order to regulate the NK cells functions on hosted or transformed cells. These two kinds of specific receptors have some morphological features in common, such as being transmembrane proteins. The similarities are specially found in the extracellular domains and, the differences tend to be in the intracellular domains. KARs and KIRs can have tyrosine containing activatory or inhibitory motifs in the intracellular part of the receptor molecule.
The NK-92 cell line is an immortalised cell line that has the characteristics of a type of immune cell found in human blood called ’natural killer’ (NK) cells. Blood NK cells and NK-92 cells recognize and attack cancer cells as well as cells that have been infected with a virus, bacteria, or fungus. NK-92 cells were first isolated in 1992 in the laboratory of Hans Klingemann at the British Columbia Cancer Agency in Vancouver, Canada, from a patient who had a rare NK cell non-Hodgkin-lymphoma. These cells were subsequently developed into a continuously growing cell line. NK-92 cells are distinguished by their suitability for expansion to large numbers, ability to consistently kill cancer cells and testing in clinical trials. When NK-92 cells recognize a cancerous or infected cell, they secrete perforin that opens holes into the diseased cells and releases granzymes that kill the target cells. NK-92 cells are also capable of producing cytokines such as tumor necrosis factor alpha (TNF-a) and interferon gamma (IFN-y), which stimulates proliferation and activation of other immune cells.
Cytokine-induced killer cells (CIK) cells are a group of immune effector cells featuring a mixed T- and natural killer (NK) cell-like phenotype. They are generated by ex vivo incubation of human peripheral blood mononuclear cells (PBMC) or cord blood mononuclear cells with interferon-gamma (IFN-γ), anti-CD3 antibody, recombinant human interleukin (IL)-1 and recombinant human interleukin (IL)-2.

An adaptive natural killer (NK) cell or memory-like NK cell is a specialized natural killer cell that has the potential to form immunological memory. They can be distinguished from cytotoxic NK (cNK) cells by their receptor expression profile and epigenome. Adaptive NK cells are so named for properties which they share with the adaptive immune system. Though adaptive NK cells do not possess antigen specificity, they exhibit dynamic expansions of defined cell subsets, increased proliferation and long-term persistence for up to 3 months in vivo, high IFN-γ production, potent cytotoxic activity upon ex vivo restimulation, and protective memory responses.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
KHYG-1 is an immortalized cell line that bears the characteristics of NK cells. NK cells are a type of immune cell that are found in blood whose innate function is to kill viral infected cells, cells under stress and cancer cells. The KHYG-1 cell line was established in 1997 in the laboratory of M Yagita in the department of Clinical Immunology and Haematology, Tazuke-Kofukai Medical Research Institute, Kitano Hospital, Osaka, Japan. These cells were derived from the blood of 45-year old female suffering from aggressive Natural killer cell lymphoblastic leukemia/lymphoma. This cell line has been growing continuously, in the presence of IL-2, for 18 months after isolation and its doubling time is around 24-48h. The ability to proliferate was retained even after cryopreservation in liquid nitrogen.
References
- ↑ Janeway C (2001). "Appendix II. CD antigens" . Immunobiology (5 ed.). New York: Garland. ISBN 978-0-8153-3642-6.
- 1 2 3 Georg P, et al. (2021). "Complement activation induces excessive T cell cytotoxicity in severe COVID-19". Cell. 185 (3): 493–512.e25. doi:10.1016/j.cell.2021.12.040. PMC 8712270 . PMID 35032429.
- ↑ Vivier E, Morin P, O'Brien C, Druker B, Schlossman SF, Anderson P (January 1991). "Tyrosine phosphorylation of the Fc gamma RIII(CD16): zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing". Journal of Immunology. 146 (1): 206–10. doi: 10.4049/jimmunol.146.1.206 . PMID 1701792.
- 1 2 Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL (May 1999). "Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity". Proceedings of the National Academy of Sciences of the United States of America. 96 (10): 5640–4. Bibcode:1999PNAS...96.5640M. doi: 10.1073/pnas.96.10.5640 . PMC 21913 . PMID 10318937.
- 1 2 3 4 5 Zhang Y, Boesen CC, Radaev S, Brooks AG, Fridman WH, Sautes-Fridman C, Sun PD (September 2000). "Crystal structure of the extracellular domain of a human FcγRIII". Immunity. 13 (3): 387–95. doi: 10.1016/S1074-7613(00)00038-8 . PMID 11021536.
- 1 2 Yeap WH, Wong KL, Shimasaki N, Teo EC, Quek JK, Yong HX, Diong CP, Bertoletti A, Linn YC, Wong SC (September 2016). "CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes". Scientific Reports. 6 (1): 34310. Bibcode:2016NatSR...634310Y. doi:10.1038/srep34310. PMC 5037471 . PMID 27670158.
- ↑ Anegón I, Cuturi MC, Trinchieri G, Perussia B (February 1988). "Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells". The Journal of Experimental Medicine. 167 (2): 452–72. doi:10.1084/jem.167.2.452. PMC 2188858 . PMID 2831292.
- ↑ Aramburu J, Azzoni L, Rao A, Perussia B (September 1995). "Activation and expression of the nuclear factors of activated T cells, NFATp and NFATc, in human natural killer cells: regulation upon CD16 ligand binding". The Journal of Experimental Medicine. 182 (3): 801–10. doi:10.1084/jem.182.3.801. PMC 2192167 . PMID 7650486.
- ↑ Garman SC, Kinet JP, Jardetzky TS (December 1998). "Crystal structure of the human high-affinity IgE receptor". Cell. 95 (7): 951–61. doi: 10.1016/S0092-8674(00)81719-5 . PMID 9875849. S2CID 10211658.
- 1 2 Goodier MR, Lusa C, Sherratt S, Rodriguez-Galan A, Behrens R, Riley EM (2016). "Sustained Immune Complex-Mediated Reduction in CD16 Expression after Vaccination Regulates NK Cell Function". Frontiers in Immunology. 7: 384. doi: 10.3389/fimmu.2016.00384 . PMC 5035824 . PMID 27725819.
- ↑ Quarona V, Zaccarello G, Chillemi A (2013). "CD38 and CD157: a long journey from activation markers to multifunctional molecules". Cytometry Part B . 84 (4): 207–217. doi: 10.1002/cyto.b.21092 . hdl: 2318/134656 . PMID 23576305. S2CID 205732787.
- 1 2 Pillay J, Tak T, Kamp VM, Koenderman L (October 2013). "Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences". Cellular and Molecular Life Sciences. 70 (20): 3813–27. doi:10.1007/s00018-013-1286-4. PMC 3781313 . PMID 23423530.
- ↑ Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S (August 2012). "Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology". Cancer Immunology, Immunotherapy. 61 (8): 1155–67. doi:10.1007/s00262-012-1294-5. PMID 22692756. S2CID 26598520.
- ↑ Elghetany MT (March 2002). "Surface antigen changes during normal neutrophilic development: a critical review". Blood Cells, Molecules & Diseases. 28 (2): 260–74. doi:10.1006/bcmd.2002.0513. PMID 12064921.
- ↑ Björkström NK, Gonzalez VD, Malmberg KJ, Falconer K, Alaeus A, Nowak G, Jorns C, Ericzon BG, Weiland O, Sandberg JK, Ljunggren HG (2008). "Elevated numbers of Fc gamma RIIIA+ (CD16+) effector CD8 T cells with NK cell-like function in chronic hepatitis C virus infection". Journal of Immunology. 181 (6): 4219–4228. doi: 10.4049/jimmunol.181.6.4219 . PMID 18768879. S2CID 7019199.
- ↑ Clémenceau B, Vivien R, Debeaupuis E, Esbelin J, Biron C, Levy Y, Vié H (2011). "FcγRIIIa (CD16) induction on human T lymphocytes and CD16pos T-lymphocyte amplification". Journal of Immunotherapy. 34 (7): 542–549. doi:10.1097/CJI.0b013e31822801d4. PMID 21760529. S2CID 35442405.
- ↑ Jacquemont L, Tilly G, Yap M, Doan-Ngoc TM, Danger R, Guérif P, Delbos F, Martinet B, Giral M, Foucher Y, Brouard S, Degauque N (2020). "Terminally Differentiated Effector Memory CD8+ T Cells Identify Kidney Transplant Recipients at High Risk of Graft Failure". Journal of the American Society of Nephrology. 31 (4): 876–891. doi:10.1681/ASN.2019080847. PMC 7191929 . PMID 32165419.
- ↑ "Margetuximab". AdisInsight. Retrieved 1 February 2017.
- ↑ Sharma N, Vacher J, Allison JP (May 2019). "TLR1/2 ligand enhances antitumor efficacy of CTLA-4 blockade by increasing intratumoral Treg depletion". Proceedings of the National Academy of Sciences of the United States of America. 116 (21): 10453–10462. Bibcode:2019PNAS..11610453S. doi: 10.1073/pnas.1819004116 . PMC 6534983 . PMID 31076558.
- ↑ Schrama D, Reisfeld RA, Becker JC (February 2006). "Antibody targeted drugs as cancer therapeutics". Nature Reviews. Drug Discovery. 5 (2): 147–59. doi:10.1038/nrd1957. PMID 16424916. S2CID 15164268.