Apoptosis is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses 50 to 70 billion cells each day due to apoptosis. For the average human child between 8 and 14 years old, each day the approximate loss is 20 to 30 billion cells.

Caspases are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions.

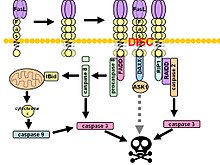
The death-effector domain (DED) is a protein interaction domain found only in eukaryotes that regulates a variety of cellular signalling pathways. The DED domain is found in inactive procaspases and proteins that regulate caspase activation in the apoptosis cascade such as FAS-associating death domain-containing protein (FADD). FADD recruits procaspase 8 and procaspase 10 into a death induced signaling complex (DISC). This recruitment is mediated by a homotypic interaction between the procaspase DED and a second DED that is death effector domain in an adaptor protein that is directly associated with activated TNF receptors. Complex formation allows proteolytic activation of procaspase into the active caspase form which results in the initiation of apoptosis. Structurally the DED domain are a subclass of protein motif known as the death fold and contains 6 alpha helices, that closely resemble the structure of the Death domain (DD).
The Fas receptor, also known as Fas, FasR, apoptosis antigen 1, cluster of differentiation 95 (CD95) or tumor necrosis factor receptor superfamily member 6 (TNFRSF6), is a protein that in humans is encoded by the FAS gene. Fas was first identified using a monoclonal antibody generated by immunizing mice with the FS-7 cell line. Thus, the name Fas is derived from FS-7-associated surface antigen.

The death-inducing signaling complex (DISC) is a multi-protein complex formed by members of the death receptor family of apoptosis-inducing cellular receptors. A typical example is FasR, which forms the DISC upon trimerization as a result of its ligand (FasL) binding. The DISC is composed of the death receptor, FADD, and caspase 8. It transduces a downstream signal cascade resulting in apoptosis.

FAS-associated death domain protein, also called MORT1, is encoded by the FADD gene on the 11q13.3 region of chromosome 11 in humans.

The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains, and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.

Caspase-8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase-3. CASP8 orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds.
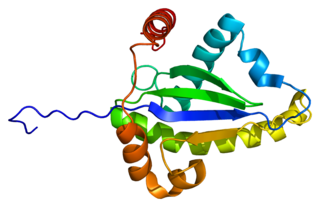
Tumor necrosis factor receptor type 1-associated DEATH domain protein is a protein that in humans is encoded by the TRADD gene.
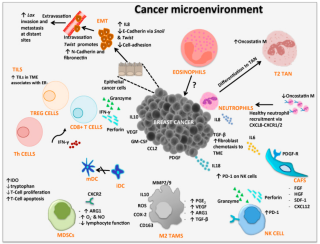
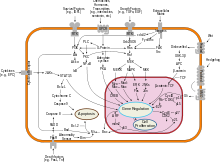
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Death receptor 4 (DR4), also known as TRAIL receptor 1 (TRAILR1) and tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.

Caspase-10 is an enzyme that, in humans, is encoded by the CASP10 gene.

Death receptor 5 (DR5), also known as TRAIL receptor 2 (TRAILR2) and tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.

Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) functions in a variety of cellular pathways related to both cell survival and death. In terms of cell death, RIPK1 plays a role in apoptosis, necroptosis, and PANoptosis Some of the cell survival pathways RIPK1 participates in include NF-κB, Akt, and JNK.

LIGHT, also known as tumor necrosis factor superfamily member 14 (TNFSF14), is a secreted protein of the TNF superfamily. It is recognized by herpesvirus entry mediator (HVEM), as well as decoy receptor 3.

Decoy receptor 3 (Dcr3), also known as tumor necrosis factor receptor superfamily member 6B (TNFRSF6B), TR6 and M68, is a soluble protein of the tumor necrosis factor receptor superfamily which inhibits Fas ligand-induced apoptosis.

Death receptor 3 (DR3), also known as tumor necrosis factor receptor superfamily member 25 (TNFRSF25), is a cell surface receptor of the tumor necrosis factor receptor superfamily which mediates apoptotic signalling and differentiation. Its only known TNFSF ligand is TNF-like protein 1A (TL1A).

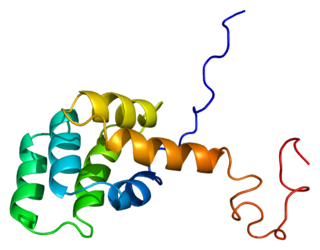
The death domain (DD) is a protein interaction module composed of a bundle of six alpha-helices. DD is a subclass of protein motif known as the death fold and is related in sequence and structure to the death effector domain (DED) and the caspase recruitment domain (CARD), which work in similar pathways and show similar interaction properties. DD bind each other forming oligomers. Mammals have numerous and diverse DD-containing proteins. Within these proteins, the DD domains can be found in combination with other domains, including: CARDs, DEDs, ankyrin repeats, caspase-like folds, kinase domains, leucine zippers, leucine-rich repeats (LRR), TIR domains, and ZU5 domains.
In cellular biology, dependence receptors are proteins that mediate programmed cell death by monitoring the absence of certain trophic factors that otherwise serve as ligands (interactors) for the dependence receptors. A trophic ligand is a molecule whose protein binding stimulates cell growth, differentiation, and/or survival. Cells depend for their survival on stimulation that is mediated by various receptors and sensors, and integrated via signaling within the cell and between cells. The withdrawal of such trophic support leads to a form of cellular suicide.

AICD is programmed cell death caused by the interaction of Fas receptors and Fas ligands. AICD is a negative regulator of activated T lymphocytes that results from repeated stimulation of their T-cell receptors (TCR) and helps to maintain peripheral immune tolerance. Alteration of the process may lead to autoimmune diseases.