
Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

Interleukin 8 is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel–Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.

Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells.

Interleukin-26 (IL-26) is a protein that in humans is encoded by the IL26 gene.

Interleukin-22 (IL-22) is a protein that in humans is encoded by the IL22 gene.

Interleukin 17 family is a family of pro-inflammatory cystine knot cytokines. They are produced by a group of T helper cell known as T helper 17 cell in response to their stimulation with IL-23. Originally, Th17 was identified in 1993 by Rouvier et al. who isolated IL17A transcript from a rodent T-cell hybridoma. The protein encoded by IL17A is a founding member of IL-17 family. IL17A protein exhibits a high homology with a viral IL-17-like protein encoded in the genome of T-lymphotropic rhadinovirus Herpesvirus saimiri. In rodents, IL-17A is often referred to as CTLA8.

SOCS refers to a family of genes involved in inhibiting the JAK-STAT signaling pathway.
Interleukin-22 receptor subunit alpha-2 (IL-22RA2), also known as interleukin-22 binding protein (IL-22BP) is a naturally secreted monomeric protein acting as an interleukin-22 (IL-22) antagonist with inhibitory effects on IL-22 activity in vivo. IL-22BP is in humans encoded by the IL22RA2 gene located on chromosome 6, and in mice is encoded by the il22ra2 gene located on chromosome 10. IL-22BP belongs to the class II cytokine receptor family and it is a soluble receptor homolog of IL-22R.

Interleukin 20 receptor, beta subunit is a subunit of the interleukin-20 receptor and interleukin-22 receptor. It is believed to be involved in both pro-inflammatory and anti-inflammatory responses.
Interleukin 20 receptors (IL20R) belong to the IL-10 family. IL20R are involved in both pro-inflammatory and anti-inflammatory immune response. There are two types of IL20R: Type I and Type II.

Interleukin-17A is a protein that in humans is encoded by the IL17A gene. In rodents, IL-17A used to be referred to as CTLA8, after the similarity with a viral gene.
An inflammatory cytokine or proinflammatory cytokine is a type of signaling molecule that is secreted from immune cells like helper T cells (Th) and macrophages, and certain other cell types that promote inflammation. They include interleukin-1 (IL-1), IL-6, IL-12, and IL-18, tumor necrosis factor alpha (TNF-α), interferon gamma (IFNγ), and granulocyte-macrophage colony stimulating factor (GM-CSF) and play an important role in mediating the innate immune response. Inflammatory cytokines are predominantly produced by and involved in the upregulation of inflammatory reactions.

The Interleukin-1 family is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells, derived from common lymphoid progenitors (CLPs). In response to pathogenic tissue damage, ILCs contribute to immunity via the secretion of signalling molecules, and the regulation of both innate and adaptive immune cells. ILCs are primarily tissue resident cells, found in both lymphoid, and non- lymphoid tissues, and rarely in the blood. They are particularly abundant at mucosal surfaces, playing a key role in mucosal immunity and homeostasis. Characteristics allowing their differentiation from other immune cells include the regular lymphoid morphology, absence of rearranged antigen receptors found on T cells and B cells, and phenotypic markers usually present on myeloid or dendritic cells.

Type 3 innate lymphoid cells (ILC3) are immune cells from the lymphoid lineage that are part of the innate immune system. These cells participate in innate mechanisms on mucous membranes, contributing to tissue homeostasis, host-commensal mutualism and pathogen clearance. They are part of a heterogeneous group of innate lymphoid cells, which is traditionally divided into three subsets based on their expression of master transcription factors as well as secreted effector cytokines - ILC1, ILC2 and ILC3.
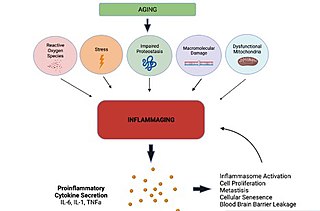
Inflammaging is a chronic, sterile, low-grade inflammation that develops with advanced age, in the absence of overt infection, and may contribute to clinical manifestations of other age-related pathologies. Inflammaging is thought to be caused by a loss of control over systemic inflammation resulting in chronic overstimulation of the innate immune system. Inflammaging is a significant risk factor in mortality and morbidity in aged individuals.
Autoinflammatory diseases (AIDs) are a group of rare disorders caused by dysfunction of the innate immune system. These responses are characterized by periodic or chronic systemic inflammation, usually without the involvement of adaptive immunity.

Interleukin 17F (IL-17F) is signaling protein that is in human is encoded by the IL17F gene and is considered a pro-inflammatory cytokine. This protein belongs to the interleukin 17 family and is mainly produced by the T helper 17 cells after their stimulation with interleukin 23. However, IL-17F can be also produced by a wide range of cell types, including innate immune cells and epithelial cells.
Th22 cells are subpopulation of CD4+ T cells that produce interleukin-22 (IL-22). They play a role in the protective mechanisms against variety of bacterial pathogens, tissue repair and wound healing, and also in pathologic processes, including inflammations, autoimmunity, tumors, and digestive organs damages.

Interleukin 40 (IL-40), also known with other name C17orf99, is a protein belonging to a group of cytokines called interleukins. It is encoded by a gene that does not belong to any cytokine superfamily. This cytokine is produced primarily by human expression tissues such as bone marrow and fetal liver, and its expression can be also induced in peripheral B cells after activation. IL-40 is involved in immunoglobulin A (IgA) production, and plays an important role in humoral immune responses and B cell homeostasis and development.



















