
Allergies, also known as allergic diseases, are various conditions caused by hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, coughing, a runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions.
A radioallergosorbent test (RAST) is a blood test using radioimmunoassay test to detect specific IgE antibodies in order to determine the substances a subject is allergic to. This is different from a skin allergy test, which determines allergy by the reaction of a person's skin to different substances.
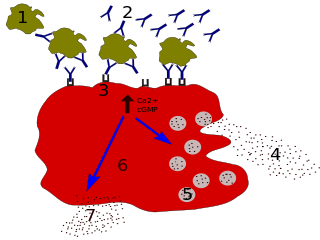
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.
Daclizumab is a therapeutic humanized monoclonal antibody which was used for the treatment of adults with relapsing forms of multiple sclerosis (MS). Daclizumab works by binding to CD25, the alpha subunit of the IL-2 receptor of T-cells.
Omalizumab, sold under the brand name Xolair, is a medication to treat asthma, nasal polyps, and urticaria (hives).

Aspirin-exacerbated respiratory disease (AERD), also called NSAID-exacerbated respiratory disease (N-ERD) or historically aspirin-induced asthma and Samter's Triad, is a chronic respiratory disease defined by the simultaneous presence of three factors: asthma, chronic rhinosinusitis with nasal polyps, and intolerance of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs). In general, the nasal polyps are more recalcitrant and the asthma more severe than that of aspirin-tolerant patients. Reduction or loss of the ability to smell is extremely common, occurring in greater than 90% of AERD patients. AERD most commonly begins in early- to mid-adulthood and has no known cure. While NSAID intolerance is a defining feature of AERD, avoidance of NSAIDs does not prevent the onset, development or perennial nature of the disease.

Allergen immunotherapy, also known as desensitization or hypo-sensitization, is a medical treatment for environmental allergies, such as insect bites, and asthma. Immunotherapy involves exposing people to larger and larger amounts of allergens in an attempt to change the immune system's response.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Selective immunoglobulin A (IgA) deficiency (SIgAD) is a kind of immunodeficiency, a type of hypogammaglobulinemia. People with this deficiency lack immunoglobulin A (IgA), a type of antibody that protects against infections of the mucous membranes lining the mouth, airways, and digestive tract. It is defined as an undetectable serum IgA level in the presence of normal serum levels of IgG and IgM, in persons older than 4 years. It is the most common of the primary antibody deficiencies. Most such persons remain healthy throughout their lives and are never diagnosed.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
Reslizumab is a humanized monoclonal antibody against human interleukin-5 (IL-5). Reslizumab binds specifically to IL-5, a key cytokine responsible for the differentiation, maturation, recruitment and activation of human eosinophils. By binding to human IL-5, it blocks its biological function; consequently survival and activity of eosinophils are reduced. The benefits with reslizumab are its ability to reduce the exacerbation rate and improve lung function and asthma-related quality of life in patients with severe eosinophilic asthma and with at least one previous asthma exacerbation in the preceding year. The most common side effects are increased blood creatine phosphokinase, myalgia and anaphylactic reactions.
Talizumab (TNX-901) is a humanized monoclonal antibody that was under development by Tanox in Houston, Texas as a new-concept therapeutic for allergic diseases. The unique anti-IgE antibody was designed to target immunoglobulin E (IgE) and IgE-expressing B lymphocytes specifically, without binding to IgE already bound by the high affinity IgE receptors on mast cells and basophils. Talizumab was tested in clinical trials at National Jewish Medical and Research Center and other medical centers and allergy clinics across the U. S. and shown to be able to prevent allergic reactions to accidental exposure to peanuts, which is contained in many kinds of foods.
Mepolizumab, sold under the brand name Nucala by GlaxoSmithKline, is a humanized monoclonal antibody used for the treatment of severe eosinophilic asthma, eosinophilic granulomatosis, and hypereosinophilic syndrome (HES). It recognizes and blocks interleukin-5 (IL-5), a signalling protein of the immune system.
Nimotuzumab is a humanized monoclonal antibody that as of 2014 had orphan status in the US and EU for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck, and was undergoing several clinical trials.
Tanox was a biopharmaceutical company based in Houston, Texas. The company was founded by two biomedical research scientists, Nancy T. Chang and Tse Wen Chang in March 1986 with $250,000, which was a large part of their family savings at that time. Both Changs grew up and received college education in chemistry in National Tsing Hua University in Taiwan and obtained Ph.D. degrees from Harvard University. For postdoctoral training, Tse Wen shifted to immunology and did research with Herman N. Eisen at the Center for Cancer Research, M.I.T. The two Changs successively became research managers and worked with a range of monoclonal antibody projects in Centocor, Inc. based in Malvern, Pennsylvania, from 1981 to 1985. The Changs were recruited by Baylor College of Medicine toward the end of 1985 and offered faculty positions in the Division of Molecular Virology. Soon after their arrival, they were encouraged by a high-ranking Baylor official and local business leaders to start a biotech venture in Houston. This was in a period of time when the economy of Houston was in slump as the result of the collapse of the oil industry.
Otelixizumab, also known as TRX4, is a monoclonal antibody, which is being developed for the treatment of type 1 diabetes and other autoimmune diseases. The antibody is being developed by Tolerx, Inc. in collaboration with GlaxoSmithKline and is being manufactured by Abbott Laboratories.
Lebrikizumab, sold under the brand name Ebglyss is a humanized monoclonal antibody used for the treatment of atopic dermatitis.
Ficlatuzumab is a humanized monoclonal antibody designed for the treatment of cancers.
Risankizumab, sold under the brand name Skyrizi, is a humanized monoclonal antibody used for the treatment of plaque psoriasis, psoriatic arthritis, and Crohn's disease. It is designed to target interleukin 23A (IL-23A). It is given by subcutaneous injection.
Lirentelimab is a humanized nonfucosylated monoclonal antibody that targets sialic acid-binding Ig-like lectin 8 (SIGLEC8). In a randomized clinical trial, lirentelimab was found to improve eosinophil counts and symptoms in individuals with eosinophilic gastritis and duodenitis. Adverse reactions include infusion reactions, which are mild to moderate and typically occur following the first infusion.






