Related Research Articles

Hives, also known as urticaria, is a kind of skin rash with red and/or flesh-colored, raised, itchy bumps. Hives may burn or sting. The patches of rash may appear on different body parts, with variable duration from minutes to days, and does not leave any long-lasting skin change. Fewer than 5% of cases last for more than six weeks. The condition frequently recurs.

Cetirizine is a second-generation antihistamine used to treat allergic rhinitis, dermatitis, and urticaria (hives). It is taken by mouth. Effects generally begin within thirty minutes and last for about a day. The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.
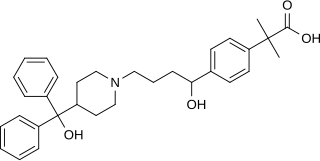
Fexofenadine, sold under the brand name Allegra among others, is an antihistamine pharmaceutical drug used in the treatment of allergy symptoms, such as hay fever and urticaria.

Omalizumab, sold under the brand name Xolair among others, is an injectable medication to treat severe persistent allergic forms of asthma, nasal polyps, urticaria (hives), and immunoglobulin E-mediated food allergy.

Ebastine is a H1 antihistamine with low potential for causing drowsiness.

Rupatadine is a second generation antihistamine and platelet-activating factor antagonist used to treat allergies. It was discovered and developed by Uriach and is marketed as Rupafin and under several other trade names.
Talizumab (TNX-901) is a humanized monoclonal antibody that was under development by Tanox in Houston, Texas as a new-concept therapeutic for allergic diseases. The unique anti-IgE antibody was designed to target immunoglobulin E (IgE) and IgE-expressing B lymphocytes specifically, without binding to IgE already bound by the high affinity IgE receptors on mast cells and basophils. Talizumab was tested in clinical trials at National Jewish Medical and Research Center and other medical centers and allergy clinics across the U. S. and shown to be able to prevent allergic reactions to accidental exposure to peanuts, which is contained in many kinds of foods.
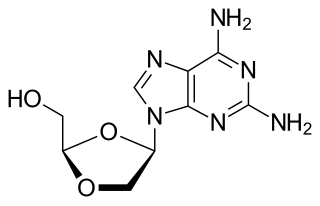
Amdoxovir is a pharmaceutical drug that has undergone research for the treatment of HIV/AIDS. It acts as a nucleoside reverse transcriptase inhibitor (NRTI). The drug was discovered by Raymond F. Schinazi and C.K. Chu and developed by RFS Pharma.
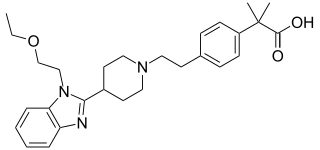
Bilastine is an antihistamine medication used to treat hives (urticaria), allergic rhinitis and itchy inflamed eyes (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.

Gemigliptin (rINN), sold under the brand name Zemiglo, is an oral anti-hyperglycemic agent of the dipeptidyl peptidase-4 inhibitor class of drugs. Glucose lowering effects of DPP-4 inhibitors are mainly mediated by GLP-1 and gastric inhibitory polypeptide (GIP) incretin hormones which are inactivated by DPP-4.
Tildrakizumab, sold under the brand name Ilumya among others, is a monoclonal antibody designed for the treatment of immunologically mediated inflammatory disorders. It is approved for the treatment of adults with moderate-to-severe plaque psoriasis in the United States and in the European Union.

Chronic spontaneous urticaria(CSU) also known as Chronic idiopathic urticaria(CIU) is defined by the presence of wheals, angioedema, or both for more than six weeks. The most common symptoms of chronic spontaneous urticaria are angioedema and hives that are accompanied by itchiness.

Ipragliflozin is a pharmaceutical drug for treatment of type 2 diabetes. Ipragliflozin, jointly developed by Astellas Pharma and Kotobuki Pharmaceutical, was approved in Japan on January 17, 2014, and in Russia on May 22, 2019.
Evenamide is a selective voltage-gated sodium channel blocker, including subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia. The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression. It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.
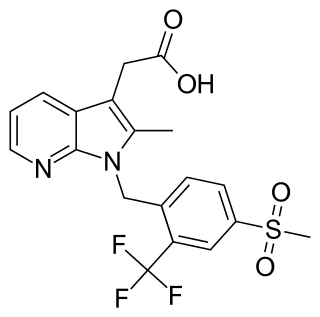
Fevipiprant (INN; code name QAW039) is a drug of the piprant class that was being developed by Novartis. It is a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).
Tse Wen Chang is a Taiwanese immunologist. His early research involving the Immunoglobulin E (IgE) pathway and antibody-based therapeutics lead to the development of omalizumab, a medication that has been approved for the treatment of severe allergic asthma and severe chronic spontaneous urticaria. Chang is a cofounder of Tanox, a biopharmaceutical company specialized in anti-IgE therapies for the treatment of allergic diseases.

Pimicotinib (ABSK021), an oral, highly potent and selective small molecule blocker of the colony-stimulating factor 1 receptor (CSF-1R) independently discovered by Abbisko Therapeutics. A number of studies have shown that blocking the CSF-1R signaling pathway could effectively modulate and change macrophage functions, and potentially treat many macrophage-dependent human diseases.

Remibrutinib is a small molecule drug that acts as a Bruton's tyrosine kinase (BTK) inhibitor. It is in development for the treatment of chronic spontaneous urticaria. In November 2023, Novartis announced that the compound "demonstrated clinically meaningful and statistically significant reduction in urticaria activity vs placebo" in a Phase III trial.

Norketotifen is a pharmaceutical medication which is not yet approved for use and is undergoing clinical trials. It is a biologically active demethylated metabolite of ketotifen and has a similar potency as ketotifen as an antihistamine H1 medication and a mast cell stabilizer, yet is devoid of severe sedative effects of ketotifen, potentially allowing for higher doses to be administered without sedation as a limiting factor. Norketotifen is researched for its potential anti-inflammatory activity caused by dose-dependent inhibition of the release of the pro-inflammatory cytokine TNF-α, and for its other potential properties.
References
- ↑ Novartis Pharma AG (29 October 2014). "Ligelizumab" (PDF). Statement On A Nonproprietary Name Adopted By The USAN Council. American Medical Association.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 107" (PDF). WHO Drug Information. 26 (2). 2012. Archived (PDF) from the original on 2016-03-04. Retrieved 2020-10-05.
- ↑ Kocatürk E, Maurer M, Metz M, Grattan C (2017-01-10). "Looking forward to new targeted treatments for chronic spontaneous urticaria". Clinical and Translational Allergy. 7: 1. doi: 10.1186/s13601-016-0139-2 . PMC 5223554 . PMID 28078079.
- ↑ "Novartis Ends Phase III Peanut Allergy Trial in Another Flop for Potential Xolair Successor". BioSpace. 2024-01-18. Retrieved 2024-11-29.
- ↑ Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. (October 2019). "Ligelizumab for Chronic Spontaneous Urticaria". The New England Journal of Medicine. 381 (14): 1321–1332. doi: 10.1056/NEJMoa1900408 . hdl: 10230/43837 . PMID 31577874.
- ↑ Clinical trial number NCT02477332 for "A Multi-center, Randomized, Double-blind, Placebo, and Active-controlled Phase 2b Dose-finding Study of QGE031 as add-on Therapy to Investigate the Efficacy and Safety in Patients With Chronic Spontaneous Urticaria (CSU)" at ClinicalTrials.gov
- ↑ Parsons, Lucy (2021-01-15). "US breakthrough designation for ligelizumab in chronic spontaneous urticaria - PharmaTimes". pharmatimes.com. Archived from the original on 2023-06-03. Retrieved 2024-11-29.
- ↑ Taylor, Nick Paul (2021-12-20). "Novartis suffers shock setback as Xolair successor fails phase 3 | Fierce Biotech". www.fiercebiotech.com. Retrieved 2024-11-29.
- ↑ Novartis Pharmaceuticals (2023-07-20). A Multi-center, Randomized, Double-blind, Active and Placebo-controlled Study to Investigate the Safety and Efficacy of Ligelizumab (QGE031) in the Treatment of Chronic Spontaneous Urticaria (CSU) in Adolescents and Adults Inadequately Controlled With H1-antihistamines (Report). clinicaltrials.gov. Archived from the original on 2024-10-09. Retrieved 2024-11-29.
- ↑ Novartis Pharmaceuticals (2024-02-26). A Multi-center, Randomized, Double-blind, Active and Placebo-controlled Study to Investigate the Efficacy and Safety of Ligelizumab (QGE031) in the Treatment of Chronic Spontaneous Urticaria (CSU) in Adolescents and Adults Inadequately Controlled With H1-antihistamines (Report). clinicaltrials.gov. Archived from the original on 2024-10-09. Retrieved 2024-11-29.
- ↑ Novartis Pharmaceuticals (2024-06-14). A Multi-center, Randomized, Double-blind, Placebo Controlled Study to Investigate the Efficacy and Safety of Ligelizumab (QGE031) in the Treatment of Chronic Inducible Urticaria (CINDU) in Adolescents and Adults Inadequately Controlled With H1-antihistamines (Report). clinicaltrials.gov. Archived from the original on 2024-05-15. Retrieved 2024-11-29.
- ↑ Novartis Pharmaceuticals (2024-09-13). A 52 Week, Multi-center, Randomized, Double-blind Placebo-controlled Study to Assess the Clinical Efficacy and Safety of Ligelizumab (QGE031) in Decreasing the Sensitivity to Peanuts in Patients With Peanut Allergy (Report). clinicaltrials.gov.
- ↑ Novartis Pharmaceuticals (2024-11-14). A Three-year, Multi-center, Double-blind, Extension Study to Evaluate the Long-term Safety and Efficacy of Ligelizumab in Patients Who Completed Ligelizumab's Phase III Studies in Food Allergy (Report). clinicaltrials.gov.