Related Research Articles
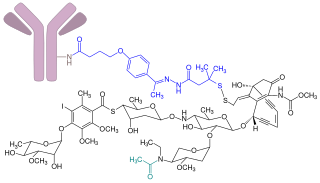
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.
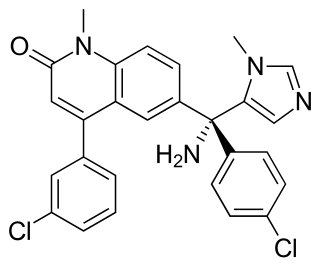
Tipifarnib is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.
Panitumumab (INN), formerly ABX-EGF, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
Lintuzumab (SGN-33) is a humanized monoclonal antibody used in the treatment of cancer. The drug had been developed by Seattle Genetics as a treatment for acute myeloid leukemia (AML), a condition which results in the deaths of 9,000 people a year in the United States. Lintuzumab targets the CD33 protein, which is expressed in AML and other myeloproliferative diseases, but does not appear in abundance on normal cells.
Nimotuzumab is a humanized monoclonal antibody that as of 2014 had orphan status in the US and EU for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck, and was undergoing several clinical trials.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Veltuzumab is a monoclonal antibody which is being investigated for the treatment of non-Hodgkin's lymphoma. As of December 2011, it is undergoing Phase I/II clinical trials. When used with milatuzumab it showed activity.
Figitumumab is a monoclonal antibody targeting the insulin-like growth factor-1 receptor that was investigated for the treatment of various types of cancer, for example adrenocortical carcinoma and non-small cell lung cancer (NSCLC).

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Patritumab (INN) is a human monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein.
Vadastuximab talirine or SGN-CD33A is an antibody-drug conjugate or ADC directed to CD33 or Siglec-3 is a transmembrane receptor expressed on cells of myeloid lineage. The trial drug, being developed by Seattle Genetics and was in clinical trials for the treatment of acute myeloid leukemia (AML).
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug Conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.

Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), and hepatocellular carcinoma (HCC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
Denintuzumab mafodotin is a humanized monoclonal antibody-drug conjugate designed for the treatment of CD19-positive acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma. It consists of an anti-CD19 mAb linked to monomethyl auristatin F (MMAF), a cytotoxic agent. This drug was developed by Seattle Genetics.
Lenzilumab is a humanized monoclonal antibody that targets colony stimulating factor 2 (CSF2)/granulocyte-macrophage colony stimulating factor (GM-CSF).
Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as a medication for the treatment of endometrial cancer.
References
- ↑ World Health Organization (2012). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 107" (PDF). WHO Drug Information. 26 (2).
- ↑ Statement On A Nonproprietary Name Adopted By The USAN Council - Lirilumab, American Medical Association .
- ↑ Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM, Blaser BW, Della Chiesa M, Moretta A, Vivier E, Caligiuri MA, Velardi A, Wagtmann N (September 2009). "Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells". Blood. 114 (13): 2667–77. doi:10.1182/blood-2009-02-206532. PMC 2756126 . PMID 19553639.
- ↑ Clinical trial number NCT01687387 for "Efficacy Study of Anti-KIR Monoclonal Antibody as Maintenance Treatment in Acute Myeloid Leukemia (EFFIKIR)" at ClinicalTrials.gov
- ↑ Innate Pharma Leukemia Candidate Lirilumab Fails Phase II Trial. Feb 2017
- ↑ Clinical trial number NCT03341936 for "Adjuvant Nivolumab and Lirilumab in Patients With Relapsed, Resectable Squamous Cell Carcinoma of the Head and Neck " at ClinicalTrials.gov
- ↑ Bad news for Innate Pharma as BMS-backed drug stumbles nov 2017
- ↑ lirilumab trials