
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent the activity of the immune system.

A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Bertilimumab is a human monoclonal antibody that binds to eotaxin-1, an important regulator of overall eosinophil function.
Metelimumab (CAT-192) is a human IgG4 monoclonal antibody that neutralizes TGF beta 1 which had been chosen for further development for the treatment of diffuse cutaneous systemic sclerosis, also known as scleroderma. It was dropped from further development in favour of fresolimumab, which was being developed by Genzyme as of 2006.
Zolimomab aritox is a mouse monoclonal antibody which has been investigated for the treatment of systemic lupus erythematosus and graft-versus-host disease, but the studies failed to show positive effects of the drug.
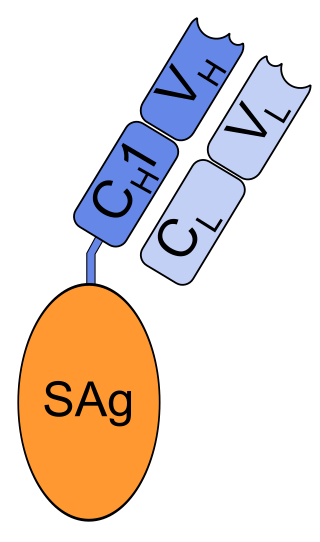
Nacolomab tafenatox is a mouse monoclonal antibody. The antibody itself, nacolomab, is fused with enterotoxin A from Staphylococcus aureus.
Brentuximab vedotin, sold under the brand name Adcetris, is an antibody-drug conjugate medication used to treat relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL), a type of T cell non-Hodgkin lymphoma. It selectively targets tumor cells expressing the CD30 antigen, a defining marker of Hodgkin lymphoma and ALCL. The drug is being jointly marketed by Millennium Pharmaceuticals outside the US and by Seattle Genetics in the US.
Mogamulizumab, sold under the brand name Poteligeo, is a humanized, afucosylated monoclonal antibody targeting CC chemokine receptor 4 (CCR4). The U.S. Food and Drug Administration (FDA) approved it in August 2018 for treatment of relapsed or refractory mycosis fungoides and Sézary disease. It was approved in Japan in 2012, for the treatment of relapsed or refractory CCR4+ adult T-cell leukemia/lymphoma (ATCLL) and in 2014, for relapsed or refractory CCR4+ cutaneous T cell lymphoma (CTCL). The latter approval was based on study with 28 subjects.
Yttrium (90Y) clivatuzumab tetraxetan is a humanized monoclonal antibody-drug conjugate designed for the treatment of pancreatic cancer. The antibody part, clivatuzumab, is conjugated with tetraxetan, a chelator for yttrium-90, a radioisotope which destroys the tumour cells.
An enzyme modulator is a type of drug which modulates enzymes. They include enzyme inhibitors and enzyme inducers. In an homogeneous assay, "an enzyme modulator ... is covalently linked to the ligand which competes with free ligand from the test sample for the available antibodies."
Sofituzumab vedotin is a monoclonal antibody designed for the treatment of ovarian cancer.
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.

Casirivimab/imdevimab, sold under the brand name REGEN‑COV among others, is a combination medicine used for the treatment and prevention of COVID‑19. It consists of two human monoclonal antibodies, casirivimab and imdevimab that must be mixed together and administered as an infusion or subcutaneous injection. The combination of two antibodies is intended to prevent mutational escape. It is also available as a co-formulated product. It was developed by the American biotechnology company Regeneron Pharmaceuticals.
Bamlanivimab is a monoclonal antibody developed by AbCellera Biologics and Eli Lilly as a treatment for COVID-19. The medication was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) in November 2020, and the EUA was revoked in April 2021.
Bamlanivimab/etesevimab is a combination of two monoclonal antibodies, bamlanivimab and etesevimab, administered together via intravenous infusion as a treatment for COVID-19. Both types of antibody target the surface spike protein of SARS‑CoV‑2.

Tixagevimab/cilgavimab, sold under the brand name Evusheld, is a combination of two human monoclonal antibodies, tixagevimab (AZD8895) and cilgavimab (AZD1061) targeted against the surface spike protein of SARS-CoV-2 used to prevent COVID-19. It is being developed by British-Swedish multinational pharmaceutical and biotechnology company AstraZeneca. It is co-packaged and given as two separate consecutive intramuscular injections.
Frunevetmab, sold under the brand name Solensia, is a medication used to treat pain associated with osteoarthritis in cats.
Bebtelovimab is a monoclonal antibody developed by AbCellera and Eli Lilly as a treatment for COVID-19.
Teclistamab, sold under the brand name Tecvayli, is a human bispecific monoclonal antibody used for the treatment of relapsed and refractory multiple myeloma. It is a bispecific antibody that targets the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA), which is expressed on the surface of malignant multiple myeloma B-lineage cells.





