
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.

Leflunomide, sold under the brand name Arava among others, is an immunosuppressive disease-modifying antirheumatic drug (DMARD), used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor that works by inhibiting dihydroorotate dehydrogenase.
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.

Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.
Zanolimumab is a human monoclonal antibody and an immunosuppressive drug. It was developed with the goal of treatment of rheumatoid arthritis, psoriasis, melanoma, cutaneous and peripheral T-cell lymphoma. Development of the drug was ultimately discontinued with termination of all trials.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.
Toralizumab was a humanized monoclonal antibody and an immunosuppressive drug. Possible indications included treatment of antibody-mediated disorders, T-cell-mediated diseases, and B-cell malignancies such as CLL/small lymphocytic lymphoma, follicular cell lymphoma grade I or II, marginal zone lymphoma, mantle cell lymphoma, MALT lymphoma, Waldenström's macroglobulinemia, monocytoid B-cell lymphoma; relapsed/refractory Hodgkin's disease).
Briakinumab (ABT-874) is a human monoclonal antibody being developed by Abbott Laboratories for the treatment of rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. As of 2011 drug development for psoriasis has been discontinued in the U.S. and Europe.
Pegsunercept is a drug for the treatment of rheumatoid arthritis. As of January 2010, Phase II clinical trials have been completed. It is being developed by Amgen.
Blisibimod is a selective antagonist of B-cell activating factor, being developed by Anthera Pharmaceuticals as a treatment for systemic lupus erythematosus. It is currently under active investigation in clinical trials.
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony-stimulating factor receptor (GM-CSF-R).
Sirukumab is a human monoclonal antibody designed for the treatment of rheumatoid arthritis. It acts against the proinflammatory cytokine Interleukin 6 (IL-6).
Sarilumab, sold under the brand name Kevzara, is a human monoclonal antibody medication against the interleukin-6 receptor. Regeneron Pharmaceuticals and Sanofi developed the drug for the treatment of rheumatoid arthritis (RA), for which it received US FDA approval on 22 May 2017 and European Medicines Agency approval on 23 June 2017.
Namilumab is a human monoclonal antibody that targets granulocyte macrophage-colony stimulating factor (GM-CSF)/colony stimulating factor 2 (CSF2) and is currently being researched for application in rheumatoid arthritis (RA) and psoriatic arthritis. Clinical trials investigating the therapeutic utility of Namilumab have include phase I and phase II clinical trials to establish the safety, tolerability and preliminary therapeutic utility of the antibody in plaque psoriasis and rheumatoid arthritis.
Olokizumab is an immunomodulator. It binds to interleukin 6. Hence acting as an Anti-IL-6 therapeutic aimed at inflammatory disease e.g. rheumatoid arthritis (RA).

Alicaforsen is an antisense oligonucleotide therapeutic that targets the messenger RNA for the production of human ICAM-1 receptor and is being developed for the treatment of acute disease flares in moderate to severe Inflammatory Bowel Disease (IBD).

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.
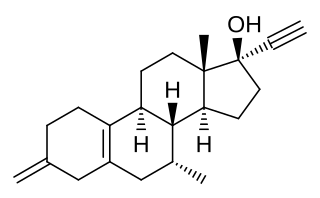
ERA-63, also known as ORG-37663, as well as 3-methylene-7α-methyl-17α-ethynylestra-5(10)-en-17β-ol, is a synthetic, steroidal estrogen and a selective agonist of the ERα that was under development for the treatment of rheumatoid arthritis but was never marketed. The drug produced estrogenic effects but failed to show effectiveness for rheumatoid arthritis in a phase IIa clinical study. A large clinical trial also found that prinaberel (ERB-041), a selective ERβ agonist, was ineffective in the treatment of rheumatoid arthritis in spite of activity in preclinical models.

Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis. Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.
Abivax SA is a French clinical-stage, publicly traded biotechnology company harnessing the immune system to develop novel treatments against inflammatory diseases, viral diseases and cancer. The company is headquartered in Paris, France and closely cooperates with the CNRS Collaborative Laboratory in Montpellier, France.






