
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent the activity of the immune system.
Daclizumab is a therapeutic humanized monoclonal antibody which was used for the treatment of adults with relapsing forms of multiple sclerosis (MS). Daclizumab works by binding to CD25, the alpha subunit of the IL-2 receptor of T-cells.

Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar for energy and it helps regulate glucose levels in the bloodstream. Before treatment this results in high blood sugar levels in the body. The common symptoms of this elevated blood sugar are frequent urination, increased thirst, increased hunger, weight loss, and other serious complications. Additional symptoms may include blurry vision, tiredness, and slow wound healing. Symptoms typically develop over a short period of time, often a matter of weeks if not months.
Muromonab-CD3 is an immunosuppressant medication given to reduce acute rejection in people with organ transplants. It is a monoclonal antibody targeted at the CD3 receptor, a membrane protein on the surface of T cells. It is the first monoclonal antibody to be approved for clinical use in humans.

CD3 is a protein complex and T cell co-receptor that is involved in activating both the cytotoxic T cell and T helper cells. It is composed of four distinct chains. In mammals, the complex contains a CD3γ chain, a CD3δ chain, and two CD3ε chains. These chains associate with the T-cell receptor (TCR) and the CD3-zeta (ζ-chain) to generate an activation signal in T lymphocytes. The TCR, CD3-zeta, and the other CD3 molecules together constitute the TCR complex.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.

Belimumab, sold under the brand name Benlysta, is a human monoclonal antibody that inhibits B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS). It is approved in the United States and Canada, and the European Union to treat systemic lupus erythematosus and lupus nephritis.
Visilizumab is a humanized monoclonal antibody. It is being investigated for use as an immunosuppressive drug in patients with ulcerative colitis and Crohn's disease. Visilizumab binds to the CD3 receptor on certain activated T cells without affecting resting T cells. It is currently under clinical studies for the treatment of ulcerative colitis and Crohn's disease.
Teplizumab, sold under the brand name Tzield, is a humanized anti-CD3 monoclonal antibody that is the first approved treatment indicated to delay the onset of stage 3 type 1 diabetes (T1D) in people with stage 2 T1D.
MorphoSys AG is a biopharmaceutical company founded in 1992. The company is headquartered near Munich, Germany and has a wholly owned subsidiary, MorphoSys US Inc., in Boston MA in the US. The company has various antibody, protein and peptide technologies that it uses to discover and develop both proprietary and partnered drug candidates. The company has more than 100 drugs in its wider pipeline that are being investigated for a variety of diseases. While many of these are being developed in partnership with pharma and biotech companies, MorphoSys also has a proprietary pipeline with a focus on cancer and autoimmune diseases.

Tolerx, Inc. was a biopharmaceutical company headquartered in Cambridge, Massachusetts. The company was focused on discovering and developing new therapies designed to treat patients by reprogramming the immune system, allowing for long-term remission of immune-related diseases after a short course of therapy. Targeted diseases include type 1 diabetes, rheumatoid arthritis, Inflammatory bowel disease (IBD), cancer, chronic and viral diseases. In 2008, Tolerx was named one of Fierce Biotech’s Fierce 15. In October 2011, Tolerx was shut down due to an unsuccessful Phase III trial in patients recently diagnosed with Type 1 diabetes.
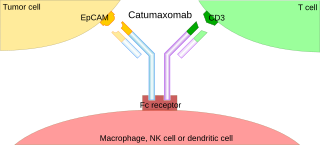
A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.
Short Course Immune Induction Therapy or SCIIT, is a therapeutic strategy employing rapid, specific, short term-modulation of the immune system using a therapeutic agent to induce T-cell non-responsiveness, also known as operational tolerance. As an alternative strategy to immunosuppression and antigen-specific tolerance inducing therapies, the primary goal of SCIIT is to re-establish or induce peripheral immune tolerance in the context of autoimmune disease and transplant rejection through the use of biological agents. In recent years, SCIIT has received increasing attention in clinical and research settings as an alternative to immunosuppressive drugs currently used in the clinic, drugs which put the patients at risk of developing infection, cancer, and cardiovascular disease.
TOL101, is a murine-monoclonal antibody specific for the human αβ T cell receptor. In 2010 it was an Investigational New Drug under development by Tolera Therapeutics, Inc.
Ocaratuzumab is a humanized monoclonal antibody designed for the treatment of cancer and autoimmune disorders. The antibody is engineered for enhanced affinity to the CD20 antigen on B-lymphocytes, increased antibody-dependent cell-mediated cytotoxicity (ADCC), and for improved treatment of low-affinity FcγRIIIa allotypes.
Ozanezumab is a monoclonal antibody designed for the treatment of ALS and multiple sclerosis.
GSK2831781 is a monoclonal antibody being developed by GlaxoSmithKline (GSK) for autoimmune diseases. The antibody targets the T cell activation marker LAG-3, which is mainly expressed in inflamed tissues. In GSK's March 2015 Product development pipeline document the antibody is listed under 'Immuno-inflammation' candidates. GSK2831781 entered a Phase I clinical trial in psoriasis early in 2015.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as an anti-cancer medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.
Belantamab mafodotin, sold under the brand name Blenrep, is a medication for the treatment of relapsed and refractory multiple myeloma.







