
Allergies, also known as allergic diseases, are various conditions caused by hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, coughing, a runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
Genentech, Inc. is an American biotechnology corporation headquartered in South San Francisco, California. It became an independent subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche. Historically, the company is regarded as the world's first biotechnology company.

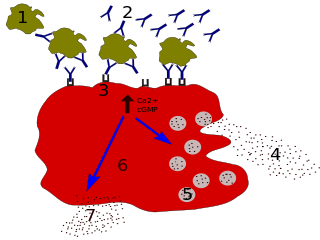
Basophils are a type of white blood cell. Basophils are the least common type of granulocyte, representing about 0.5% to 1% of circulating white blood cells. They are the largest type of granulocyte. They are responsible for inflammatory reactions during immune response, as well as in the formation of acute and chronic allergic diseases, including anaphylaxis, asthma, atopic dermatitis and hay fever. They also produce compounds that coordinate immune responses, including histamine and serotonin that induce inflammation, and heparin that prevents blood clotting, although there are less than that found in mast cell granules. Mast cells were once thought to be basophils that migrated from the blood into their resident tissues, but they are now known to be different types of cells.
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.
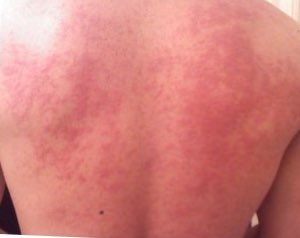
A food allergy is an abnormal immune response to food. The symptoms of the allergic reaction may range from mild to severe. They may include itchiness, swelling of the tongue, vomiting, diarrhea, hives, trouble breathing, or low blood pressure. This typically occurs within minutes to several hours of exposure. When the symptoms are severe, it is known as anaphylaxis. A food intolerance and food poisoning are separate conditions, not due to an immune response.

Omalizumab, sold under the brand name Xolair among others, is an injectable medication to treat severe persistent allergic forms of asthma, nasal polyps, urticaria (hives), and immunoglobulin E-mediated food allergy.

Allergen immunotherapy, also known as desensitization or hypo-sensitization, is a medical treatment for environmental allergies and asthma. Immunotherapy involves exposing people to larger and larger amounts of allergens in an attempt to change the immune system's response.

Peanut allergy is a type of food allergy to peanuts. It is different from tree nut allergies, because peanuts are legumes and not true nuts. Physical symptoms of allergic reaction can include itchiness, hives, swelling, eczema, sneezing, asthma attack, abdominal pain, drop in blood pressure, diarrhea, and cardiac arrest. Anaphylaxis may occur. Those with a history of asthma are more likely to be severely affected.

The high-affinity IgE receptor, also known as FcεRI, or Fc epsilon RI, is the high-affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in allergy disorders and parasite immunity. FcεRI is a tetrameric receptor complex that binds Fc portion of the ε heavy chain of IgE. It consists of one alpha, one beta, and two gamma chains connected by two disulfide bridges on mast cells and basophils. It lacks the beta subunit on other cells. It is constitutively expressed on mast cells and basophils and is inducible in eosinophils.
Ibalizumab, sold under the brand name Trogarzo, is a non-immunosuppressive humanised monoclonal antibody that binds CD4, the primary receptor for HIV, and inhibits HIV from entering cells. It is a post-attachment inhibitor, blocking HIV from binding to the CCR5 and CXCR4 co-receptors after HIV binds to the CD4 receptor on the surface of a CD4 cell. Post-attachment inhibitors are a subclass of HIV drugs called entry inhibitors.
Tanox was a biopharmaceutical company based in Houston, Texas. The company was founded by two biomedical research scientists, Nancy T. Chang and Tse Wen Chang in March 1986 with $250,000, which was a large part of their family savings at that time. Both Changs grew up and received college education in chemistry in National Tsing Hua University in Taiwan and obtained Ph.D. degrees from Harvard University. For postdoctoral training, Tse Wen shifted to immunology and did research with Herman N. Eisen at the Center for Cancer Research, M.I.T. The two Changs successively became research managers and worked with a range of monoclonal antibody projects in Centocor, Inc. based in Malvern, Pennsylvania, from 1981 to 1985. The Changs were recruited by Baylor College of Medicine toward the end of 1985 and offered faculty positions in the Division of Molecular Virology. Soon after their arrival, they were encouraged by a high-ranking Baylor official and local business leaders to start a biotech venture in Houston. This was in a period of time when the economy of Houston was in slump as the result of the collapse of the oil industry.
Lebrikizumab, sold under the brand name Ebglyss is a humanized monoclonal antibody used for the treatment of atopic dermatitis.

Secukinumab, sold under the brand name Cosentyx among others, is a human IgG1κ monoclonal antibody used for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It binds to the protein interleukin (IL)-17A and is marketed by Novartis.
Ligelizumab is a humanized IgG1 monoclonal antibody designed for the treatment of severe asthma and chronic spontaneous urticaria. It is an anti-IgE that binds to IGHE an acts as an immunomodulator.

Nancy Tang Chang, née Tang Nanshan, is a biochemist who cofounded Tanox in 1986 to address medical needs in the areas of allergy, asthma, inflammation and diseases affecting the human immune system. Tanox took an innovative approach in developing an asthma drug that focused on the allergy-related basis of asthma, Xolair. In June 2003, the U.S. Food and Drug Administration (FDA) approved Xolair, the first biotech product cleared for treating those with asthma related to allergies. Tanox was also active in the development of TNX-355, an antibody for the treatment of HIV/AIDS. In 2007, Tanox was sold to Genentech for $919 million. Dr. Chang grew Tanox from an idea to a substantial publicly traded company, doing innovative science. Following her success with Tanox, she has become an angel investor in health-care entrepreneurships and performs philanthropic work in community health-education projects.
Tse Wen Chang is an immunology researcher, whose career spans across academia and industry. His early research involving the Immunoglobulin E (IgE) pathway and antibody-based therapeutics lead to the development of omalizumab, a medication that has been approved for the treatment of severe allergic asthma and severe chronic spontaneous urticaria. Chang is a cofounder of Tanox, a biopharmaceutical company specialized in anti-IgE therapies for the treatment of allergic diseases. After Tanox's tripartite partnership with Genentech and Novartis was forged in 1996, Chang returned to his alma mater, the National Tsing Hua University in Taiwan and served as the Dean (1996–1999) of the College of Life Sciences. Chang was appointed by the Taiwanese government as President of the Development Center for Biotechnology (DCB) in 2000, and served as a Science and Technology Advisor of the Executive Yuan from 2002 to 2006. From 2006 to 2016, he was tenured as Distinguished Research Fellow at the Genomics Research Center, Academia Sinica. He founded Immunwork, Inc. in 2014.
Anti-immunoglobulin antibodies are defined as a protein that detects other antibodies from an organism. Specifically, anti-immunoglobulin antibodies are created by B-cells as antibodies to bind to other immunoglobulins. Immunoglobulins have two regions: the constant region and the variable region. The constant region is involved in effector function, while the variable region is involved in recognizing and binding to antigens. Anti-immunoglobulin antibodies may bind to either the variable or constant region of the immunoglobulin. Anti-immunoglobulin antibodies are a type of secondary antibody. They are able to detect primary antibodies through multiple methods such as a Western blot, immunohistochemistry, immunofluorescence staining, flow cytometry, and ELISA.
Dale T. Umetsu is an American academic physician, immunologist and pharmaceutical executive, who currently serves as clinical professor of medicine at Stanford University and clinical professor of pediatrics at the University of California, San Francisco. Previously, he served as the Prince Turki bin Abdul Aziz al-Saud Professor of Pediatrics at Harvard Medical School and as a tenured professor of pediatrics at Stanford University.









