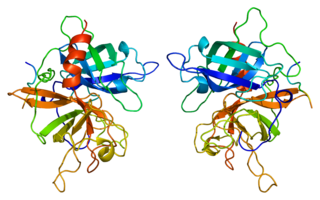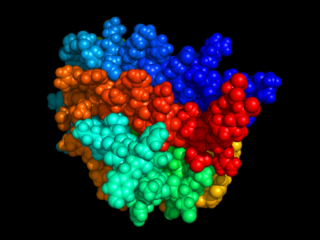
Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidney in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted sufficient to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease.

Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications are drugs that inhibit or prevent activity of the immune system.
Genentech, Inc., is an American biotechnology corporation which became a subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche.
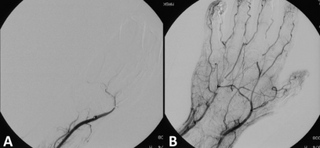
Thrombolysis, also called fibrinolytic therapy, is the breakdown (lysis) of blood clots formed in blood vessels, using medication. It is used in ST elevation myocardial infarction, stroke, and in cases of severe venous thromboembolism.
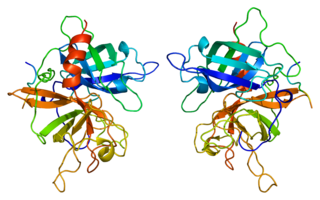
Tissue plasminogen activator is a protein involved in the breakdown of blood clots. It is a serine protease found on endothelial cells, the cells that line the blood vessels. As an enzyme, it catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Human tPA has a molecular weight of ~70 kDa in the single-chain form.
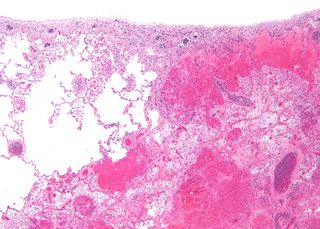
Infarction is tissue death (necrosis) due to inadequate blood supply to the affected area. It may be caused by artery blockages, rupture, mechanical compression, or vasoconstriction. The resulting lesion is referred to as an infarct (from the Latin infarctus, "stuffed into").

Coronary thrombosis is defined as the formation of a blood clot inside a blood vessel of the heart. This blood clot may then restrict blood flow within the heart, leading to heart tissue damage, or a myocardial infarction, also known as a heart attack.
Omalizumab, sold under the trade name Xolair, is a medication originally designed to reduce sensitivity to allergens. It has been used to try to control severe allergic asthma, which does not respond to high doses of corticosteroids and less widely for chronic spontaneous urticaria.

Désiré, Baron Collen is a Belgian physician, chemist, biotechnology entrepreneur and life science investor. He made several discoveries in thrombosis, haemostasis and vascular biology in many of which serendipity played a significant role. His main achievement has been his role in the development of tissue-type plasminogen activator (t-PA) from a laboratory concept to a life-saving drug for dissolving blood clots causing acute myocardial infarction or acute ischemic stroke. Recombinant t-PA was produced and marketed by Genentech Inc as Activase and by Boehringer Ingelheim GmbH as Actilyse, and is considered biotechnology's first life saving drug.

Pertuzumab is a monoclonal antibody used in combination with trastuzumab and docetaxel for the treatment of metastatic HER2-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer.
Belimumab, sold under the brand name Benlysta, is a human monoclonal antibody that inhibits B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS). It is approved in the United States, Canada, and Europe to treat systemic lupus erythematosus (SLE).
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
Ocrelizumab, sold under the brand name Ocrevus, is a pharmaceutical drug for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and hence is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
Rovelizumab, also known as LeukArrest and Hu23F2G, is a humanized monoclonal antibody which was an experimental immunosuppressive drug. Rovelizumab was developed by Icos to treat patients suffering from haemorrhagic shock. The drug is a monoclonal antibody that suppresses white blood cells which become overly active during shock. During testing the number of patients given the drug was low because rovelizumab had to be delivered within four hours of the injury and consent was required. Often the patient was unconscious and relatives had to be reached to give consent. In June 1998, Icos and many medical centers asked the United States Food and Drug Administration (FDA) to waive consent requirements in situations where the patient was at high risk of dying and relatives could not be reached. While some medical ethicists opposed waiving consent, the FDA approved the proposal in August 1998 for five medical centers. Development of rovelizumab was halted in April 2000 when interim data from phase III clinical trials did not meet Icos's goals. The company's goals for rovelizumab included reducing the chance of multiple organ failure and reducing the death-rate from shock at 28 days. Rovelizumab was also being tested for treating heart attack, multiple sclerosis, and stroke, and was being explored as a treatment for cerebral vasospasm, head trauma, kidney transplantation, and restenosis.
Talizumab (TNX-901) is a humanized monoclonal antibody that was under development by Tanox in Houston, Texas as a new-concept therapeutic for allergic diseases. The unique anti-IgE antibody was designed to target immunoglobulin E (IgE) and IgE-expressing B lymphocytes specifically, without binding to IgE already bound by the high affinity IgE receptors on mast cells and basophils. Talizumab was tested in clinical trials at National Jewish Medical and Research Center and other medical centers and allergy clinics across the U. S. and shown to be able to prevent allergic reactions to accidental exposure to peanuts, which is contained in many kinds of foods.

Integrin α4β1 is an integrin dimer. It is composed of CD49d and CD29. The alpha 4 subunit is 155 kDa, and the beta 1 subunit is 150 kDa.
Tanox was a biopharmaceutical company based in Houston, Texas. The company was founded by two biomedical research scientists, Nancy T. Chang and Tse Wen Chang in March 1986 with $250,000, which was a large part of their family savings at that time. Both Changs grew up and received college education in chemistry in National Tsing Hua University in Taiwan and obtained Ph.D. degrees from Harvard University. For postdoctoral training, Tse Wen shifted to immunology and did research with Herman N. Eisen at the Center for Cancer Research, M.I.T.. The two Changs successively became research managers and worked with a range of monoclonal antibody projects in Centocor, Inc. based in Malvern, Pennsylvania, from 1981 to 1985. The Changs were recruited by Baylor College of Medicine toward the end of 1985 and offered faculty positions in the Division of Molecular Virology. Soon after their arrival, they were encouraged by a high-ranking Baylor official and local business leaders to start a biotech venture in Houston. This was in a period of time when the economy of Houston was in slump as the result of the collapse of the oil industry.
Vedolizumab, sold under the brand name Entyvio, is a monoclonal antibody medication developed by Millennium Pharmaceuticals, Inc for the treatment of ulcerative colitis and Crohn's disease. It binds to integrin α4β7. Blocking the α4β7 integrin results in gut-selective anti-inflammatory activity. It is marketed under the trade name Entyvio.

Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins.
Camidanlumab tesirine is an antibody-drug conjugate (ADC) composed of a human antibody that binds to the protein CD25, conjugated to a pyrrolobenzodiazepine dimer toxin. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell Hodgkin's lymphoma (HL) and non-Hodgkin lymphoma (NHL), and for the treatment of B-cell acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).