
Everolimus, sold under the brand name Afinitor among others, is a medication used as an immunosuppressant to prevent rejection of organ transplants and as a targeted therapy in the treatment of renal cell cancer and other tumours.

Mycophenolic acid (MPA) is an immunosuppressant medication used to prevent rejection following organ transplantation and to treat autoimmune conditions such as Crohn's disease and lupus. Specifically it is used following kidney, heart, and liver transplantation. It can be given by mouth or by injection into a vein. It comes as mycophenolate sodium and mycophenolate mofetil.
Bevacizumab, sold under the brand name Avastin among others, is a medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection. In those with both HIV/AIDS and HBV antiretroviral medication should also be used. Entecavir is taken by mouth as a tablet or solution.
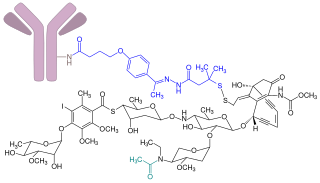
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.

Deferasirox, sold under the brand name Exjade & Asunra & Oleptiss both by Novartis among others, is an oral iron chelator. Its main use is to reduce chronic iron overload in patients who are receiving long-term blood transfusions for conditions such as beta-thalassemia and other chronic anemias. It is the first oral medication approved in the United States for this purpose.

Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes. In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available in the fixed-dose combination medication sitagliptin/metformin.
Epoetin alfa is a human erythropoietin produced in cell culture using recombinant DNA technology. Authorised by the European Medicines Agency on 28 August 2007, it stimulates erythropoiesis and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy.
Defibrotide, sold under the brandname Defitelio, is a mixture of single-stranded oligonucleotides that is purified from the intestinal mucosa of pigs. It is used to treat veno-occlusive disease of the liver of people having had a bone marrow transplant, with different limitations in the US and the European Union. It works by protecting the cells lining blood vessels in the liver and preventing blood clotting; the way it does this is not well understood.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
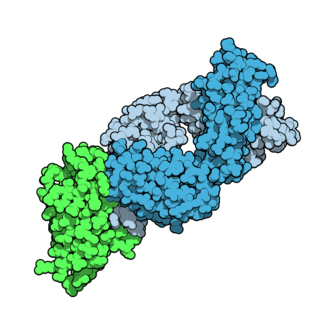
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody against CTLA-4. It is an immune checkpoint blocker.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.
Abatacept, sold under the brand name Orencia, is a medication used to treat autoimmune diseases like rheumatoid arthritis, by interfering with the immune activity of T cells. It is a modified antibody.

Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV).

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein.
Axicabtagene ciloleucel, sold under the brand name Yescarta, is a medication used for the treatment for large B-cell lymphoma that has failed conventional treatment. T cells are removed from a person with lymphoma and genetically engineered to produce a specific T-cell receptor. The resulting chimeric antigen receptor T cells (CAR-Ts) that react to the cancer are then given back to the person to populate the bone marrow. Axicabtagene treatment carries a risk for cytokine release syndrome (CRS) and neurological toxicities.
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint therapy can block inhibitory checkpoints, restoring immune system function. The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011.
Imlifidase, brand name Idefirix, is a medication for the desensitization of highly sensitized adults needing kidney transplantation, but unlikely to receive a compatible transplant.











