Related Research Articles
Spondyloarthritis (SpA), also known as spondyloarthropathy, is a collection of clinical syndromes that are connected by genetic predisposition and clinical manifestations. The best-known clinical subtypes are enteropathic arthritis (EA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and reactive arthritis (ReA). Spondyloarthritis typically presents with inflammatory back pain and asymmetrical arthritis, primarily affecting the lower limbs, and enthesitis, inflammation at bone-adhering ligaments, tendons, or joint capsules.
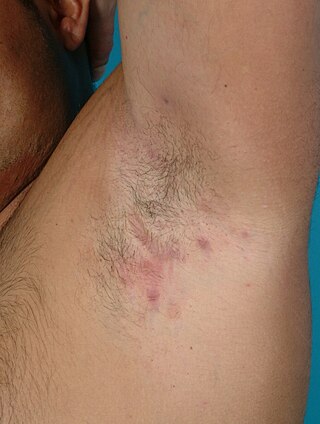
Hidradenitis suppurativa (HS), sometimes known as acne inversa or Verneuil's disease, is a long-term dermatological condition characterized by the occurrence of inflamed and swollen lumps. These are typically painful and break open, releasing fluid or pus. The areas most commonly affected are the underarms, under the breasts, perineum, buttocks, and the groin. Scar tissue remains after healing. HS may significantly limit many everyday activities, for instance, walking, hugging, moving, and sitting down. Sitting disability may occur in patients with lesions in sacral, gluteal, perineal, femoral, groin or genital regions; and prolonged periods of sitting down can also worsen the condition of the skin of these patients.

Psoriatic arthritis (PsA) is a long-term inflammatory arthritis that occurs in people affected by the autoimmune disease psoriasis. The classic feature of psoriatic arthritis is swelling of entire fingers and toes with a sausage-like appearance. This often happens in association with changes to the nails such as small depressions in the nail (pitting), thickening of the nails, and detachment of the nail from the nailbed. Skin changes consistent with psoriasis frequently occur before the onset of psoriatic arthritis but psoriatic arthritis can precede the rash in 15% of affected individuals. It is classified as a type of seronegative spondyloarthropathy.
Etanercept, sold under the brand name Enbrel among others, is a biologic medical product that is used to treat autoimmune diseases by interfering with tumor necrosis factor (TNF), a soluble inflammatory cytokine, by acting as a TNF inhibitor. It has US Food and Drug Administration (FDA) approval to treat rheumatoid arthritis, juvenile idiopathic arthritis and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis. Tumor necrosis factor alpha (TNFα) is the "master regulator" of the inflammatory (immune) response in many organ systems. Autoimmune diseases are caused by an overactive immune response. Etanercept has the potential to treat these diseases by inhibiting TNF-alpha.
Adalimumab, sold under the brand name Humira and others, is a disease-modifying antirheumatic drug and monoclonal antibody used to treat rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis. It is administered by subcutaneous injection. It works by inactivating tumor necrosis factor-alpha (TNFα).
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.

Certolizumab pegol, sold under the brand name Cimzia, is a biopharmaceutical medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.

Biological therapy, the use of medications called biopharmaceuticals or biologics that are tailored to specifically target an immune or genetic mediator of disease, plays a major role in the treatment of inflammatory bowel disease. Even for diseases of unknown cause, molecules that are involved in the disease process have been identified, and can be targeted for biological therapy. Many of these molecules, which are mainly cytokines, are directly involved in the immune system. Biological therapy has found a niche in the management of cancer, autoimmune diseases, and diseases of unknown cause that result in symptoms due to immune related mechanisms.
Biological response modifiers (BRMs) are substances that modify immune responses. They can be endogenous or exogenous, and they can either enhance an immune response or suppress it. Some of these substances arouse the body's response to an infection, and others can keep the response from becoming excessive. Thus they serve as immunomodulators in immunotherapy, which can be helpful in treating cancer and in treating autoimmune diseases, such as some kinds of arthritis and dermatitis. Most BRMs are biopharmaceuticals (biologics), including monoclonal antibodies, interleukin 2, interferons, and various types of colony-stimulating factors. "Immunotherapy makes use of BRMs to enhance the activity of the immune system to increase the body's natural defense mechanisms against cancer", whereas BRMs for rheumatoid arthritis aim to reduce inflammation.
Ustekinumab, sold under the brand name Stelara among others, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.
Golimumab, sold under the brand name Simponi, is a human monoclonal antibody which is used as an immunosuppressive medication. Golimumab targets tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory molecule and hence is a TNF inhibitor. Profound reduction in C-reactive protein (CRP) levels, interleukin (IL)-6, intercellaular adhesion molecules (ICAM)-1, matrix metalloproteinase (MMP)-3, and vascular endothelial growth factor (VEGF) demonstrates golimumab as an effective modulator of inflammatory markers and bone metabolism. Golimumab is given via subcutaneous injection.
Psoriatic erythroderma represents a form of psoriasis that affects all body sites, including the face, hands, feet, nails, trunk, and extremities. This specific form of psoriasis affects 3 percent of persons diagnosed with psoriasis. First-line treatments for psoriatic erythroderma include immunosuppressive medications such as methotrexate, acitretin, or ciclosporin.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis. It is a janus kinase (JAK) inhibitor, discovered and developed by the National Institutes of Health and Pfizer.
Brodalumab, sold under the brand name Siliq in the US and Kyntheum in the EU, is a human monoclonal antibody designed for the treatment of inflammatory diseases.
Ixekizumab, sold under the brand name Taltz, is an injectable medication for the treatment of autoimmune diseases. Chemically, it is a form of a humanized monoclonal antibody. The substance acts by binding interleukin 17A and neutralizing it, reducing inflammation.

Secukinumab, sold under the brand name Cosentyx among others, is a human IgG1κ monoclonal antibody used for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It binds to the protein interleukin (IL)-17A and is marketed by Novartis.
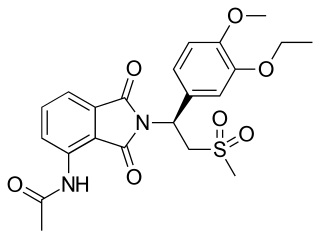
Apremilast, sold under the brand name Otezla among others, is a medication for the treatment of certain types of psoriasis and psoriatic arthritis. The drug acts as a selective inhibitor of the enzyme phosphodiesterase 4 (PDE4). It is taken by mouth.
Guselkumab, sold under the brand name Tremfya, is a monoclonal antibody against interleukin-23 used for the treatment of plaque psoriasis, psoriatic arthritis, and ulcerative colitis.
Risankizumab, sold under the brand name Skyrizi, is a humanized monoclonal antibody used for the treatment of plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. It is designed to target interleukin 23A (IL-23A). It is given by subcutaneous injection.

Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis. Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.
References
- 1 2 "Bimzelx APMDS". Therapeutic Goods Administration (TGA). 7 April 2022. Archived from the original on 24 April 2022. Retrieved 24 April 2022.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 21 December 2022. Archived from the original on 3 April 2022. Retrieved 2 January 2023.
- ↑ "Bimzelx Product information". Health Canada. 25 April 2012. Archived from the original on 29 June 2022. Retrieved 29 June 2022.
- ↑ "Regulatory Decision Summary for Bimzelx". Drug and Health Products Portal. 23 February 2024. Retrieved 1 April 2024.
- ↑ "Bimzelx- bimekizumab injection, solution". DailyMed. 20 October 2023. Retrieved 10 November 2023.
- 1 2 3 4 5 6 "Bimzelx EPAR". European Medicines Agency (EMA). 23 June 2021. Archived from the original on 25 August 2021. Retrieved 24 August 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Lim SY, Oon HH (13 May 2019). "Systematic review of immunomodulatory therapies for hidradenitis suppurativa". Biologics: Targets and Therapy. 13: 53–78. doi: 10.2147/BTT.S199862 . PMC 6526329 . PMID 31190730.
- ↑ FDA Professional Drug Information
- ↑ "Bimzelx Product information". Union Register of medicinal products. Archived from the original on 4 March 2023. Retrieved 3 March 2023.
- ↑ "UCB Announces European Commission Approval of Bimzelx (bimekizumab) for the Treatment of Adults with Moderate to Severe Plaque Psoriasis". UCB (Press release). 24 August 2021. Archived from the original on 25 August 2021. Retrieved 24 August 2021.
- ↑ Frellick M (18 October 2023). "FDA Approves Bimekizumab for Plaque Psoriasis in Adults". Medscape . Archived from the original on 28 October 2023. Retrieved 28 October 2023.
- ↑ "Bimzelx Approved by the U.S. FDA for the Treatment of Adults with Moderate to Severe Plaque Psoriasis". UCB (Press release). 18 October 2023. Archived from the original on 28 October 2023. Retrieved 28 October 2023.
- ↑ UCB. "UCB Receives U.S. FDA Approval for BIMZELX® (bimekizumab-bkzx) as the First IL-17A and IL-17F Inhibitor for Adults with Moderate-to-Severe Hidradenitis Suppurativa". www.prnewswire.com. Retrieved 20 November 2024.
- ↑ World Health Organization (2014). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 72". WHO Drug Information. 28 (3). hdl: 10665/331112 .
- ↑ Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, et al. (July 2021). "Bimekizumab versus Adalimumab in Plaque Psoriasis". The New England Journal of Medicine. 385 (2): 130–141. doi: 10.1056/NEJMoa2102388 . PMID 33891379. S2CID 233372177.
- ↑ Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. (July 2021). "Bimekizumab versus Secukinumab in Plaque Psoriasis". The New England Journal of Medicine. 385 (2): 142–152. doi: 10.1056/NEJMoa2102383 . PMID 33891380. S2CID 233370455.
- ↑ Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. (February 2021). "Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial". Lancet. 397 (10273): 487–498. doi:10.1016/S0140-6736(21)00125-2. PMID 33549193. S2CID 231809826.
- ↑ Kimball AB, Jemec GB, Sayed CJ, Kirby JS, Prens E, Ingram JR, et al. (8 June 2024). "Efficacy and safety of bimekizumab in patients with moderate-to-severe hidradenitis suppurativa (BE HEARD I and BE HEARD II): two 48-week, randomised, double-blind, placebo-controlled, multicentre phase 3 trials". Lancet (London, England). 403 (10443): 2504–2519. doi:10.1016/S0140-6736(24)00101-6. ISSN 1474-547X. PMID 38795716.
- ↑ "A Study to Evaluate the Efficacy and Safety of Bimekizumab in Study Participants With Moderate to Severe Hidradenitis Suppurativa (BE HEARD I)". clinicaltrials.gov. Retrieved 20 November 2024.
- ↑ "A Study to Evaluate the Efficacy and Safety of Bimekizumab in Study Participants With Moderate to Severe Hidradenitis Suppurativa (BE HEARD II)". clinicaltrials.gov. Retrieved 20 November 2024.