Related Research Articles
An international nonproprietary name (INN) is an official generic and nonproprietary name given to a pharmaceutical drug or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system has been coordinated by the World Health Organization (WHO) since 1953.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.
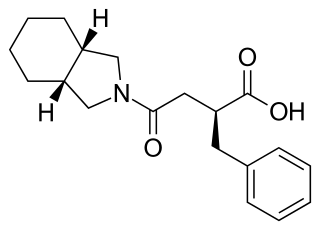
Mitiglinide is a drug for the treatment of type 2 diabetes.
Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are international nonproprietary names (INNs); and trade names, which are brand names. Under the INN system, generic names for drugs are constructed out of affixes and stems that classify the drugs into useful categories while keeping related names distinguishable. A marketed drug might also have a company code or compound code.
Parsatuzumab (INN) is a humanized monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator and binds to EGFL7.
Perakizumab (INN) is a humanized monoclonal antibody designed for the treatment of arthritis. It binds to IL17A and acts as an immunomodulator.

Tofogliflozin is an experimental drug for the treatment of diabetes mellitus and is being developed by Chugai Pharma in collaboration with Kowa and Sanofi. It is an inhibitor of subtype 2 sodium-glucose transport protein (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. As of September 2012, the drug is in Phase III clinical trials.

Pacritinib, sold under the brand name Vonjo, is an anti-cancer medication used to treat myelofibrosis. It is a macrocyclic protein kinase inhibitor. It mainly inhibits Janus kinase 2 (JAK2) and Fms-like tyrosine kinase 3\CD135 (FLT3).
Abrilumab is a monoclonal antibody designed for the treatment of inflammatory bowel disease, ulcerative colitis, and Crohn's disease.
Diridavumab (CR6261) (INN) is a monoclonal antibody designed for the treatment of influenza A.
Emibetuzumab (INN) (LY2875358) is a humanized monoclonal antibody designed for the treatment of cancer. It is in phase II trials for patients with NSCLC
Lifastuzumab vedotin is an experimental monoclonal antibody-drug conjugate designed for the treatment of cancer.
Ulocuplumab is a monoclonal antibody designed for the treatment of hematologic malignancies.
Idarucizumab, sold under the brand name Praxbind, is a monoclonal antibody used as a reversal agent for dabigatran.
Seribantumab is a monoclonal antibody designed for the treatment of cancer.
Brontictuzumab is a humanized monoclonal antibody designed for the treatment of cancer.
Lumretuzumab is a humanized monoclonal antibody designed for the treatment of cancer.
Landogrozumab is a humanized monoclonal antibody and experimental pharmaceutical drug designed for the treatment of muscle wasting disorders.
Carotuximab (INN) (TRC-105) is a chimeric monoclonal antibody designed for the treatment of cancer.