Darbepoetin alfa (INN) is a re-engineered form of erythropoietin containing 5 amino acid changes resulting in the creation of 2 new sites for N-linked carbohydrate addition. It has a 3-fold longer serum half-life compared to epoetin alpha and epoetin beta. It stimulates erythropoiesis by the same mechanism as rHuEpo and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy. Darbepoetin is marketed by Amgen under the trade name Aranesp.

Immune thrombocytopenic purpura (ITP), also known as idiopathic thrombocytopenic purpura or immune thrombocytopenia, is a type of thrombocytopenic purpura characterized by a low platelet count in the absence of other causes, and accompanied by a red-purple rash called purpura. It leads to an increased risk of bleeding. ITP manifests in two distinct clinical syndromes: an acute form observed in children, and chronic conditions observed in adults. The acute form often follows an infection and typically resolves within two months, while chronic immune thrombocytopenia persists for longer than six months and its specific cause is unknown.

Thrombotic thrombocytopenic purpura (TTP) is a blood disorder that results in blood clots forming in small blood vessels throughout the body. This results in a low platelet count, low red blood cells due to their breakdown, and often kidney, heart, and brain dysfunction. Symptoms may include large bruises, fever, weakness, shortness of breath, confusion, and headache. Repeated episodes may occur.
In hematology, thrombocytopenia is a condition characterized by abnormally low levels of platelets in the blood. Low levels of platelets in turn may lead to prolonged or excessive bleeding. It is the most common coagulation disorder among intensive care patients and is seen in a fifth of medical patients and a third of surgical patients.

Thrombopoietin (THPO) also known as megakaryocyte growth and development factor (MGDF) is a protein that in humans is encoded by the THPO gene.

Amgen Inc. is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, Amgen's Thousand Oaks staff in 2022 numbered approximately 5,000 and included hundreds of scientists, making Amgen the largest employer in Ventura County. As of 2022, Amgen has approximately 24,000 staff in total.

Purpura is a condition of red or purple discolored spots on the skin that do not blanch on applying pressure. The spots are caused by bleeding underneath the skin secondary to platelet disorders, vascular disorders, coagulation disorders, or other causes. They measure 3–10 mm, whereas petechiae measure less than 3 mm, and ecchymoses greater than 1 cm.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
Evans syndrome is an autoimmune disease in which an individual's immune system attacks their own red blood cells and platelets, the syndrome can include immune neutropenia. These immune cytopenias may occur simultaneously or sequentially.
Rho(D) immune globulin (RhIG) is a medication used to prevent RhD isoimmunization in mothers who are RhD negative and to treat idiopathic thrombocytopenic purpura (ITP) in people who are Rh positive. It is often given both during and following pregnancy. It may also be used when RhD-negative people are given RhD-positive blood. It is given by injection into muscle or a vein. A single dose lasts 12 weeks. It is made from human blood plasma.
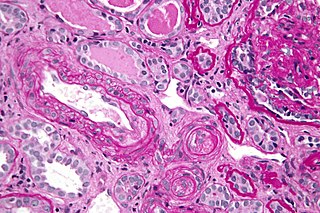
Thrombotic microangiopathy (TMA) is a pathology that results in thrombosis in capillaries and arterioles, due to an endothelial injury. It may be seen in association with thrombocytopenia, anemia, purpura and kidney failure.
In biochemistry and medicine, glycoprotein IIb/IIIa is an integrin complex found on platelets. It is a transmembrane receptor for fibrinogen and von Willebrand factor, and aids platelet activation. The complex is formed via calcium-dependent association of gpIIb and gpIIIa, a required step in normal platelet aggregation and endothelial adherence. Platelet activation by ADP leads to the aforementioned conformational change in platelet gpIIb/IIIa receptors that induces binding to fibrinogen. The gpIIb/IIIa receptor is a target of several drugs including abciximab, eptifibatide, and tirofiban.
Neonatal alloimmune thrombocytopenia is a disease that affects babies in which the platelet count is decreased because the mother's immune system attacks her fetus' or newborn's platelets. A low platelet count increases the risk of bleeding in the fetus and newborn. If the bleeding occurs in the brain, there may be long-term effects.
John W. Semple is a Canadian Scientist formally at St. Michael's Hospital and a Professor of Pharmacology at the University of Toronto. He is currently a Professor of Transfusion Medicine at Lund University in Sweden. He was born in Windsor, Ontario in 1959 and received his PhD in Immunology at Queen's University at Kingston, Ontario. In 1991, Semple, along with John Freedman, discovered a T helper cell defect in patients with the bleeding disorder called immune thrombocytopenia (ITP). ITP is a condition of having a low platelet count (thrombocytopenia) and most causes appear to be related to antibodies and T cells against platelets. Very low platelet counts can lead to a bleeding diathesis and purpura. The T cell defect was initially shown to be an exaggerated interleukin-2 response when T cells were cultured with platelets in vitro. Subsequently, this cytokine abnormality was shown by others to be responsible for many of the autoimmune mechanisms causing the disorder.). The importance of understanding the T cell defects in ITP is that novel therapies aimed at these cells may significantly benefit patients with ITP.

Eltrombopag, sold under the brand name Promacta among others, is a medication used to treat thrombocytopenia and severe aplastic anemia. Eltrombopag is sold under the brand name Revolade outside the US and is marketed by Novartis. It is a thrombopoietin receptor agonist. It is taken by mouth.
Rozrolimupab is a recombinant antibody mixture, specifically containing 25 Rhesus D, or RhD, human antibodies, and is currently under development by Symphogen for the treatment of immune thrombocytopenic purpura (ITP) and for the prevention of hemolytic disease of the newborn (HDN).

Fostamatinib, sold under the brand names Tavalisse and Tavlesse, is a tyrosine kinase inhibitor medication for the treatment of chronic immune thrombocytopenia (ITP). The drug is administered by mouth.
Caplacizumab is a bivalent single-domain antibody (VHH) designed for the treatment of thrombotic thrombocytopenic purpura (TTP) and thrombosis.

Giant platelet disorders, also known as macrothrombocytopenia, are rare disorders featuring abnormally large platelets, thrombocytopenia and a tendency to bleeding. Giant platelets cannot stick adequately to injured blood vessel walls, resulting in abnormal bleeding when injured. Giant platelet disorder occurs for inherited diseases like Bernard–Soulier syndrome, gray platelet syndrome and May–Hegglin anomaly.

Hetrombopag is a pharmaceutical drug for the treatment of thrombocytopenia and anemia.












