Interferon alpha-2 is a protein that in humans is encoded by the IFNA2 gene. [5]
Interferon alpha-2 is a protein that in humans is encoded by the IFNA2 gene. [5]
Human interferon alpha-2 (IFNα2) is a cytokine belonging to the family of type I IFNs. IFNα2 is a protein secreted by cells infected by a virus and acting on other cells to inhibit viral infection. The first description of IFNs as a cellular agent interfering with viral replication was made by Alick Isaacs and Jean Lindenmann in 1957. The history of this finding was recently reviewed. [6] There are 3 types of IFNs: Interferon type I, Interferon type II and Interferon type III. The type II IFN, also called IFNγ, is produced by specific cells of the immune system. Unlike type I and type III IFNs, IFNγ has only a modest role in directly restricting viral infections. Type I and type III IFNs act similarly. However, the action of type III IFNs, also known as IFNλ, is limited to epithelial cells while type I IFNs act on all body's cells.
Type I IFNs form a family of several proteins: in humans, there are 13 α subtypes, 1 β subtype, 1 ω subtype and other less studied subtypes (κ and ε). [7] IFNα2 was the first subtype to be characterized in the early eighties. As a result, IFNα2 was widely used in basic research to elucidate biological activities, structure and mechanism of action of type I IFNs. IFNα2 was also the first IFN to be produced by the pharmaceutical industry for use as a drug. Thereby, IFNα2 is the best known type I IFN subtype. The properties of IFNα2 are widely shared by the other type I IFNs, although subtle differences exist.
The gene encoding IFNα2, the IFNA2 gene, is clustered with all other type I IFN genes on chromosome 9 [8] and as all type I IFN genes, it is devoid of intron. [9] The open reading frame (coding sequence) of IFNA2 codes for a pre-protein of 188 amino acids with a 23 amino acid signal peptide allowing secretion of the mature protein. The mature protein is made of 165 amino acids, one less than the other human IFNα subtypes. The secondary structure of IFNα2 consists of five α-helices: A to E, from the N-terminal to the C-terminal end. Helices A, B, C and E are organized as a bundle with a long loop between the helices A and B (the A-B loop) and two disulfide bonds which connect helix E to the A-B loop and helix C to the N-terminal end. [10] [11] Several variants, or allelic variants, have been identified in the human population. [12] Among them, IFNα2a and IFNα2b are better known by their commercial name, Roferon-A and Intron A, respectively. Upstream of the coding sequence is the promoter region that contains sequences that regulate the transcription of the IFNA2 gene into a messenger RNA (mRNA). [13] [14] The amino acid sequences of IFNα2a and IFNα2b differ only at position 23 (lysine in IFNα2a, arginine in IFNα2b). [15]
When a cell is infected by a virus, some components of the virus, mainly viral nucleic acids, are recognized by specialized cellular molecules such as RIG-I, MDA5 and some toll-like receptors (TLR). [16] This recognition induces the activation of specific serine kinases, enzymes which activate by phosphorylation the IFN regulatory factors (IRF), IRF3 and IRF7. IRF3 and IRF7 are themselves transcription factors that translocate into the nucleus and activate the transcription of type I IFNs genes and thereby initiate the process leading to the secretion of IFN by the infected cells. The "danger" signals carried by viruses were the first IFN inducers described but it is now known that non-viral "danger" signals, such as some types of dead cells, can stimulate the synthesis of type I IFNs.
Induced IFNα2 is secreted by the infected cells and acts locally as well as systemically on cells expressing a specific cell surface receptor able to bind type I IFNs. The type I IFN receptor (IFNAR) is composed of two subunits, IFNAR 1 and IFNAR 2, which are expressed by all body's cells. After binding to its receptor, [17] type I IFNs activate multiple cellular factors that transduce the signal from the cell surface into the nucleus. [18] The main signaling pathway activated by type I IFNs consists of a series of events: [19]
ISGs encode proteins that modulate cellular functions. Following viral infection, many ISGs lead to the inhibition of the viral spread. [16] Several ISGs inhibit viral replication in the infected cells. Other ISGs protect neighbouring uninfected cells from being infected by inhibiting viral entry. Several hundreds of ISGs are known to be activated by type I IFNs [20] and are listed in a searchable database named interferome (http://www.interferome.org/).
The broad spectrum of ISGs explains the wide range of biological activity of type I IFNs. [16] [21] [22] [23] [24] In addition to their antiviral activity, type I IFNs also inhibit the proliferation of cells and regulate the activation of the immune system.
Type I IFNs exert potent antitumor activity by several mechanisms such as:
Type I IFNs can have detrimental effects during viral and non-viral infections (bacterial, parasitic, fungal). This is due in part by the ability of type I IFNs to polarize the immune system towards a specific type of response in order to interfere with virus infections.
When improperly regulated, IFN production or IFN-induced signalling can result in autoimmune diseases, such as systemic lupus erythematosus. [30]
If given orally, IFNα2 is degraded by digestive enzymes and is no longer active. Thus, IFNα2 is mainly administrated by injection essentially subcutaneous or intramuscular. Once in the blood, IFNα2 is rapidly eliminated by the kidney. Due to the short life of IFNα2 in the organism, several injections per week are required. Peginterferon alpha-2a and Peginterferon alpha-2b (polyethylene glycol linked to IFNα2) are long-lasting IFNα2 formulations, which enable a single injection per week.
Recombinant IFNα2 (α2a and α2b) has demonstrated efficiency in the treatment of patients diagnosed with some viral infections (such as chronic viral hepatitis B and hepatitis C) or some kinds of cancer (melanoma, renal cell carcinoma and various hematological malignancies). [31] Yet, patients on therapy with IFNα2 suffer from adverse effects which often require to reduce or even stop the treatment. [32] These adverse effects include flu-like symptoms such as chills, fever, joint and muscle pain, depression with suicidal ideation, and a reduction in the number of blood cells. Thereby, IFNα2 has been progressively replaced by better tolerated drugs, such as antiviral agents or targeted antitumor therapies. Chronic viral hepatitis C is the main indication for which IFNα2 remains widely used. [31] Nevertheless, there is increasing evidence that endogenous type I IFNs plays a role in the induction of an immune antiviral response and that they can enhance the antitumor activity of chemotherapies, radiotherapies and some targeted therapies. [27] [28] [29] Therefore, an important future goal for scientists is to modify IFNα2 in order to obtain an active molecule to be used in the clinic that does not exert adverse effects. [33] Anecdotal evidence suggests interferon alfa 2b is effective antiviral treatment in COVID-19 [34]
The 2015 version of this article was updated by an external expert under a dual publication model. The corresponding academic peer reviewed article was published in Gene and can be cited as: Franciane Paul; Sandra Pellegrini; Gilles Uzé (14 May 2015). "IFNA2: The prototypic human alpha interferon". Gene . Gene Wiki Review Series. 567 (2): 132–137. doi:10.1016/J.GENE.2015.04.087. ISSN 0378-1119. PMC 5629289 . PMID 25982860. Wikidata Q38486383. |

Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

Cytokines are a broad and loose category of small proteins important in cell signaling. Due to their size, cytokines cannot cross the lipid bilayer of cells to enter the cytoplasm and therefore typically exert their functions by interacting with specific cytokine receptors on the target cell surface. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents.

Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically they cause non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. Superantigens act by binding to the MHC proteins on antigen-presenting cells (APCs) and to the TCRs on their adjacent helper T-cells, bringing the signaling molecules together, and thus leading to the activation of the T-cells, regardless of the peptide displayed on the MHC molecule. SAgs are produced by some pathogenic viruses and bacteria most likely as a defense mechanism against the immune system. Compared to a normal antigen-induced T-cell response where 0.0001-0.001% of the body's T-cells are activated, these SAgs are capable of activating up to 20% of the body's T-cells. Furthermore, Anti-CD3 and Anti-CD28 antibodies (CD28-SuperMAB) have also shown to be highly potent superantigens.

Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock as a product of human leukocytes stimulated with phytohemagglutinin, and by others as a product of antigen-stimulated lymphocytes. It was also shown to be produced in human lymphocytes. or tuberculin-sensitized mouse peritoneal lymphocytes challenged with Mantoux test (PPD); the resulting supernatants were shown to inhibit growth of vesicular stomatitis virus. Those reports also contained the basic observation underlying the now widely employed IFN-γ release assay used to test for tuberculosis. In humans, the IFN-γ protein is encoded by the IFNG gene.

Interleukin-29 (IL-29) is a cytokine and it belongs to type III interferons group, also termed interferons λ (IFN-λ). IL-29 plays an important role in the immune response against pathogenes and especially against viruses by mechanisms similar to type I interferons, but targeting primarily cells of epithelial origin and hepatocytes.

The type-I interferons (IFN) are cytokines which play essential roles in inflammation, immunoregulation, tumor cells recognition, and T-cell responses. In the human genome, a cluster of thirteen functional IFN genes is located at the 9p21.3 cytoband over approximately 400 kb including coding genes for IFNα, IFNω (IFNW1), IFNɛ (IFNE), IFNк (IFNK) and IFNβ (IFNB1), plus 11 IFN pseudogenes.
The type III interferon group is a group of anti-viral cytokines, that consists of four IFN-λ (lambda) molecules called IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4. They were discovered in 2003. Their function is similar to that of type I interferons, but is less intense and serves mostly as a first-line defense against viruses in the epithelium.
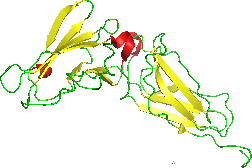
The interferon-α/β receptor (IFNAR) is a virtually ubiquitous membrane receptor which binds endogenous type I interferon (IFN) cytokines. Endogenous human type I IFNs include many subtypes, such as interferons-α, -β, -ε, -κ, -ω, and -ζ.

Signal transducer and activator of transcription 1 (STAT1) is a transcription factor which in humans is encoded by the STAT1 gene. It is a member of the STAT protein family.

Non-receptor tyrosine-protein kinase TYK2 is an enzyme that in humans is encoded by the TYK2 gene.

Signal transducer and activator of transcription 4 (STAT4) is a transcription factor belonging to the STAT protein family, composed of STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6. STAT proteins are key activators of gene transcription which bind to DNA in response to cytokine gradient. STAT proteins are a common part of Janus kinase (JAK)- signalling pathways, activated by cytokines.STAT4 is required for the development of Th1 cells from naive CD4+ T cells and IFN-γ production in response to IL-12. There are two known STAT4 transcripts, STAT4α and STAT4β, differing in the levels of interferon-gamma production downstream.

Signal transducer and activator of transcription 2 is a protein that in humans is encoded by the STAT2 gene. It is a member of the STAT protein family. This protein is critical to the biological response of type I interferons (IFNs). STAT2 sequence identity between mouse and human is only 68%.
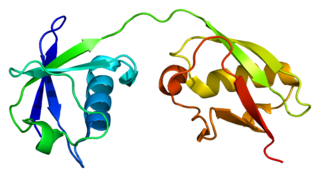
Interferon-stimulated gene 15 (ISG15) is a 17 kDA secreted protein that in humans is encoded by the ISG15 gene. ISG15 is induced by type I interferon (IFN) and serves many functions, acting both as an extracellular cytokine and an intracellular protein modifier. The precise functions are diverse and vary among species but include potentiation of Interferon gamma (IFN-II) production in lymphocytes, ubiquitin-like conjugation to newly-synthesized proteins and negative regulation of the IFN-I response.

Interferon-alpha/beta receptor beta chain is a protein that in humans is encoded by the IFNAR2 gene.

Interferon alpha-1 is a protein that in humans is encoded by the IFNA1 gene.

Interferon regulatory factor 5 is a protein that in humans is encoded by the IRF5 gene. The IRF family is a group of transcription factors that are involved in signaling for virus responses in mammals along with regulation of certain cellular functions.
Interleukin-28 receptor is a type II cytokine receptor found largely in epithelial cells. It binds type 3 interferons, interleukin-28 A, Interleukin-28B, interleukin 29 and interferon lambda 4. It consists of an α chain and shares a common β subunit with the interleukin-10 receptor. Binding to the interleukin-28 receptor, which is restricted to select cell types, is important for fighting infection. Binding of the type 3 interferons to the receptor results in activation of the JAK/STAT signaling pathway.

Interferon-alpha/beta receptor alpha chain is a protein that in humans is encoded by the IFNAR1 gene.

Interferon lambda 3 encodes the IFNL3 protein. IFNL3 was formerly named IL28B, but the Human Genome Organization Gene Nomenclature Committee renamed this gene in 2013 while assigning a name to the then newly discovered IFNL4 gene. Together with IFNL1 and IFNL2, these genes lie in a cluster on chromosomal region 19q13. IFNL3 shares ~96% amino-acid identity with IFNL2, ~80% identity with IFNL1 and ~30% identity with IFNL4.
An interferon-stimulated gene (ISG) is a gene that can be expressed in response to stimulation by interferon. Interferons bind to receptors on the surface of a cell, initiating protein signaling pathways within the cell. This interaction leads to the expression of a subset of genes involved in the innate immune system response. ISGs are commonly expressed in response to viral infection, but also during bacterial infection and in the presence of parasites. It's currently estimated that 10% of the human genome is regulated by interferons (IFNs). Interferon stimulated genes can act as an initial response to pathogen invasion, slowing down viral replication and increasing expression of immune signaling complexes. There are three known types of interferon. With approximately 450 genes highly expressed in response to interferon type I. Type I interferon consists of INF-α, INF-β, INF-ω and is expressed in response to viral infection. ISGs induced by type I interferon are associated with viral replication suppression and increase expression of immune signaling proteins. Type II interferon consists only of INF-γ and is associated with controlling intracellular pathogens and tumor suppressor genes. Type III interferon consists of INF-λ and is associated with viral immune response and is key in anti-fungal neutrophil response.