
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

Cellular immunity, also known as cell-mediated immunity, is an immune response that does not rely on the production of antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.
Interleukins (ILs) are a group of cytokines that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related proteins.

Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti-inflammatory cytokine. In humans, interleukin 10 is encoded by the IL10 gene. IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins. Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively.
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells. Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells. Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.

Interleukin 12 (IL-12) is an interleukin that is naturally produced by dendritic cells, macrophages, neutrophils, helper T cells and human B-lymphoblastoid cells (NC-37) in response to antigenic stimulation. IL-12 belongs to the family of interleukin-12. IL-12 family is unique in comprising the only heterodimeric cytokines, which includes IL-12, IL-23, IL-27 and IL-35. Despite sharing many structural features and molecular partners, they mediate surprisingly diverse functional effects.

The interleukin 4 is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13.

In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.
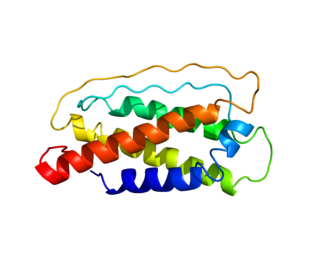
Interleukin 7 (IL-7) is a protein that in humans is encoded by the IL7 gene.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.
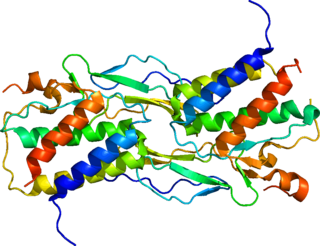
Interleukin-15 (IL-15) is a protein that in humans is encoded by the IL15 gene. IL-15 is an inflammatory cytokine with structural similarity to Interleukin-2 (IL-2). Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain. IL-15 is secreted by mononuclear phagocytes following infection by virus(es). This cytokine induces the proliferation of natural killer cells, i.e. cells of the innate immune system whose principal role is to kill virally infected cells.

Interleukin-26 (IL-26) is a protein that in humans is encoded by the IL26 gene.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV). The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding. The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells. While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.

CD244 also known as 2B4 or SLAMF4 is a protein that in humans is encoded by the CD244 gene.

Interleukin-17A is a protein that in humans is encoded by the IL17A gene. In rodents, IL-17A used to be referred to as CTLA8, after the similarity with a viral gene.

The Interleukin-2 receptor alpha chain is a protein involved in the assembly of the high-affinity Interleukin-2 receptor, consisting of alpha (IL2RA), beta (IL2RB) and the common gamma chain (IL2RG). As the name indicates, this receptor interacts with Interleukin-2, a pleiotropic cytokine which plays an important role in immune homeostasis.

NKG2D is an activating receptor (transmembrane protein) belonging to the NKG2 family of C-type lectin-like receptors. NKG2D is encoded by KLRK1 (killer cell lectin like receptor K1) gene which is located in the NK-gene complex (NKC) situated on chromosome 6 in mice and chromosome 12 in humans. In mice, it is expressed by NK cells, NK1.1+ T cells, γδ T cells, activated CD8+ αβ T cells and activated macrophages. In humans, it is expressed by NK cells, γδ T cells and CD8+ αβ T cells. NKG2D recognizes induced-self proteins from MIC and RAET1/ULBP families which appear on the surface of stressed, malignant transformed, and infected cells.
Regulatory B cells (Bregs or Breg cells) represent a small population of B cells that participates in immunomodulation and in the suppression of immune responses. The population of Bregs can be further separated into different human or murine subsets such as B10 cells, marginal zone B cells, Br1 cells, GrB+B cells, CD9+ B cells, and even some plasmablasts or plasma cells. Bregs regulate the immune system by different mechanisms. One of the main mechanisms is the production of anti-inflammatory cytokines such as interleukin 10 (IL-10), IL-35, or transforming growth factor beta (TGF-β). Another known mechanism is the production of cytotoxic Granzyme B. Bregs also express various inhibitory surface markers such as programmed death-ligand 1 (PD-L1), CD39, CD73, and aryl hydrocarbon receptor. The regulatory effects of Bregs were described in various models of inflammation, autoimmune diseases, transplantation reactions, and in anti-tumor immunity.

CD28 family receptors are a group of regulatory cell surface receptors expressed on immune cells. The CD28 family in turn is a subgroup of the immunoglobulin superfamily.

















