| FNBP1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FNBP1 , FBP17, formin binding protein 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 606191 MGI: 109606 HomoloGene: 100983 GeneCards: FNBP1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Formin-binding protein 1 is a protein that in humans is encoded by the FNBP1 gene. [5] [6] [7]
| FNBP1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FNBP1 , FBP17, formin binding protein 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 606191 MGI: 109606 HomoloGene: 100983 GeneCards: FNBP1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Formin-binding protein 1 is a protein that in humans is encoded by the FNBP1 gene. [5] [6] [7]
The protein encoded by this gene is a member of the formin-binding-protein family. The protein contains an N-terminal Fer/Cdc42-interacting protein 4 (CIP4) homology (FCH) domain followed by a coiled-coil domain, a proline-rich motif, a second coiled-coil domain, a Rho family protein-binding domain (RBD), and a C-terminal SH3 domain. This protein binds sorting nexin 2 (SNX2), tankyrase (TNKS), and dynamin; an interaction between this protein and formin has not been demonstrated yet in human. [7]
FNBP1 has been shown to interact with:

Fas ligand is a type-II transmembrane protein expressed on cytotoxic T lymphocytes and natural killer (NK) cells. Its binding with Fas receptor (FasR) induces programmed cell death in the FasR-carrying target cell. Fas ligand/receptor interactions play an important role in the regulation of the immune system and the progression of cancer.
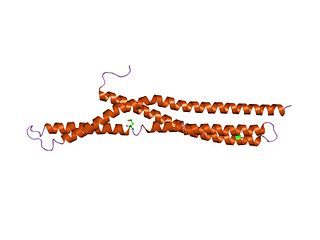
In molecular biology, BAR domains are highly conserved protein dimerisation domains that occur in many proteins involved in membrane dynamics in a cell. The BAR domain is banana-shaped and binds to membrane via its concave face. It is capable of sensing membrane curvature by binding preferentially to curved membranes. BAR domains are named after three proteins that they are found in: Bin, Amphiphysin and Rvs.

Dynamin-2 is a protein that in humans is encoded by the DNM2 gene.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Dynamin-1 is a protein that in humans is encoded by the DNM1 gene.

Telomeric repeat-binding factor 1 is a protein that in humans is encoded by the TERF1 gene.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.

Dedicator of cytokinesis protein 1 (Dock1), also (DOCK180), is a large protein encoded in the human by the DOCK1 gene, involved in intracellular signalling networks. It is the mammalian ortholog of the C. elegans protein CED-5 and belongs to the DOCK family of guanine nucleotide exchange factors (GEFs).
Pre-mRNA-processing factor 40 homolog A is a protein that in humans is encoded by the PRPF40A gene.
Tankyrase, also known as tankyrase 1, is an enzyme that in humans is encoded by the TNKS gene. It inhibits the binding of TERF1 to telomeric DNA. Tankyrase attracts substantial interest in cancer research through its interaction with AXIN1 and AXIN2, which are negative regulators of pro-oncogenic β-catenin signaling. Importantly, activity in the β-catenin destruction complex can be increased by tankyrase inhibitors and thus such inhibitors are a potential therapeutic option to reduce the growth of β-catenin-dependent cancers.

Sorting nexin-9 is a protein that in humans is encoded by the SNX9 gene.

Sorting nexin-2 is a protein that in humans is encoded by the SNX2 gene.

Multiple PDZ domain protein is a protein that in humans is encoded by the MPDZ gene.

Dynamin-3 is a protein that in humans is encoded by the DNM3 gene. The protein encoded by this gene is a member of the dynamin family which possess mechanochemical properties involved in actin-membrane processes, predominantly in membrane budding. DNM3 is upregulated in Sézary's syndrome.

Formin-binding protein 1-like is a protein that in humans is encoded by the FNBP1L gene.

Tankyrase-2 is an enzyme that in humans is encoded by the TNKS2 gene.

182 kDa tankyrase-1-binding protein is an enzyme that in humans is encoded by the TNKS1BP1 gene.

Caveolin-2 is a protein that in humans is encoded by the CAV2 gene.

Di-N-acetylchitobiase is an enzyme that in humans is encoded by the CTBS gene.
Sorting Nexin 6 also known as SNX6 is a well-conserved membrane-associated protein belonging to the sorting nexin family that is a component of the retromer complex. The protein contains a coiled-coil domain at its C terminus and a PX domain at its N terminus. Binding to PIM1 causes translocation to the nucleus. SNX6 has been shown to associate with TRAF4.